| Structure Identification: |
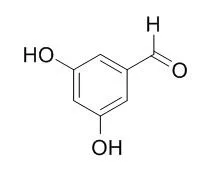
| Rapid Commun Mass Spectrom, 2010, 24(5):634-642. | | Liquid chromatographic/electrospray ionization mass spectrometric identification of the oxidation end-products of trans -resveratrol in aqueous solutions.[Reference: WebLink] | trans-Resveratrol (3,5,4'-trihydroxystilbene) is a natural polyphenolic compound that exhibits antioxidant properties. Our study aimed at studying the HO*-induced oxidation of resveratrol (100 micromol.L(-1)) in aerated aqueous solutions.
METHODS AND RESULTS:
Gamma radiolysis of water was used to generate HO*/O(2)(*-) free radicals (I = 10 Gy.min(-1), dose = 400 Gy). Oxidation products were identified by direct infusion mass spectrometry and high-performance liquid chromatography/mass spectrometry. For each product, structural elucidation was based on simple mass spectra, fragmentation spectra and deuterium/hydrogen exchange spectra; the comparison with mass spectra of synthetic products provided valuable information allowing the complete identification of the oxidation products.
CONCLUSIONS:
Four products resulting from the direct attack of HO* radicals towards resveratrol were identified respectively as piceatannol (trans-3,5,3',4'-tetrahydroxystilbene), 3,5-dihydroxybenzoic acid, 3,5-Dihydroxybenzaldehyde and 4-hydroxybenzaldehyde. | | Indian Journal of Pharmaceutical Sciences,73,1(2011-11-11), 2011, 73(1):46-56. | | Stability-indicating HPLC Method for Simultaneous Determination of Terbutaline Sulphate, Bromhexine Hydrochloride and Guaifenesin.[Reference: WebLink] | The aim of the present study was the development and subsequent validation of a simple, precise and stability-indicating reversed phase HPLC method for the simultaneous determination of guaifenesin, terbutaline sulphate and bromhexine hydrochloride in the presence of their potential impurities in a single run.
METHODS AND RESULTS:
The photolytic as well as hydrolytic impurities were detected as 3,5-dihydroxybenzoic acid, 3,5-Dihydroxybenzaldehyde, 1-(3,5-dihydroxyphenyl)-2-[(1,1-dimethylethyl) amino]-ethanone from terbutaline, 2-methoxyphenol and an unknown impurity identified as (2RS)-3-(2-hydroxyphenoxy)-propane-1,2-diol from guaifenesin. The chromatographic separation of all the three active components and their impurities was achieved on Wakosil II column, using phosphate buffer (pH 3.0) and acetonitrile as mobile phase which was delivered initially in the ratio of 80:20 (v/v) for 18 min, then changed to 60:40 (v/v) for next 12 min, and finally equilibrated back to 80:20 (v/v) for 10 min. Other HPLC parameters were: Flow rate at 1.0 ml/min, detection wavelengths 248 and 280 nm, injection volume 10 μl. The calibration graphs plotted with five concentrations of each component were linear with a regression coefficient R(2) >0.9999. The limit of detection and limit of quantitation were estimated for all the five impurities. The established method was then validated for linearity, precision, accuracy, and specificity and demonstrated to be applicable to the determination of the active ingredients in commercial and model cough syrup. No interference from the formulation excipients was observed.
CONCLUSIONS:
These results suggest that this LC method can be used for the determination of multiple active ingredients and their impurities in a cough and cold syrup. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)