| Kinase Assay: |
| BioResources,2016,11(3):6232-43. | | Experimental and Theoretical Studies on the Pyrolysis Mechanism of β-1-Type Lignin Dimer Model Compound[Reference: WebLink] | A β-1-type lignin dimer, 1,2-bis(3,5-dimethoxyphenyl)propane-1,3-diol was employed as a model compound in this study.
METHODS AND RESULTS:
The pyrolysis mechanisms and formation pathways of the pyrolytic products were investigated by using density functional theory (DFT) calculations and analytical pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS). Four possible initial pyrolysis mechanisms were proposed, including the Cα-Cβ homolysis mechanism and three concerted decomposition mechanisms (1, 2, and 3). Results indicated that the lignin dimer decomposed via two concerted decomposition mechanisms, forming 3,5-dimethoxybenzaldehyde, 1,3-dimethoxy-5-vinylbenzene, 3-hydroxy-5-methoxybenzaldehyde, and 3-methoxybenzaldehyde. 3,5-Dimethoxybenzaldehyde was the major product, accounting for greater than 50% of all pyrolytic products.
CONCLUSIONS:
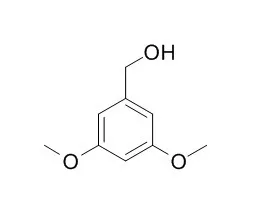
In addition to the two concerted decomposition mechanisms, Cα-Cβ homolysis was a secondary pyrolysis mechanism during the lignin dimer pyrolysis process, and the pyrolytic products included 3,5-Dimethoxybenzylalcohol, 3,5-dimethoxyphenethyl alcohol, 1,3-dimethoxybenzene, and 1,3-dimethoxy-5-methylbenzene.
A third concerted decomposition mechanism was judged to be the least likely pathway to occur because of the high activation energy requirement. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)