| In vitro: |
| Z Naturforsch C. 2008 Nov-Dec;63(11-12):794-800. | | Identification of ellagic acid derivatives in methanolic extracts from Qualea species.[Pubmed: 19227825] |
METHODS AND RESULTS:
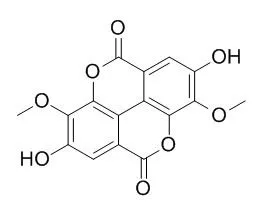
The methanolic extract from the barks of the medicinal plant Qualea parviflora (Vochysiaceae) was fractionated by column chromatography over silica gel followed by gel permeation over Sephadex LH-20 to give 3,3'-di-O-methylellagic acid-4-O-beta-D-glucopyranoside (1), 3-O-methylellagic acid-4'-O-alpha-L-rhamnopyranoside (2), 3,3',4-tri-O-methylellagic acid-4'-O-beta-D-glucopyranoside (3), and 3,3'-di-O-methylellagic acid (3,8-Di-O-methylellagic acid,4), together with triterpenes and saponins. We also performed comparative analyses among this species and Q. grandiflora and Q. multiflora using high-pressure liquid chromatography.
CONCLUSIONS:
The biological assays showed that, when compared to the standard ellagic acid, compounds 1-4 are less cytotoxic but have a lower capacity of stimulating murine peritoneal macrophages to release nitric oxide and tumoural-alpha necrose factor. | | J Agric Food Chem. 2008 Dec 24;56(24):11668-74. | | Active compounds from Lagerstroemia speciosa, insulin-like glucose uptake-stimulatory/inhibitory and adipocyte differentiation-inhibitory activities in 3T3-L1 cells.[Pubmed: 19053366 ] |
METHODS AND RESULTS:
Seven ellagitannins, lagerstroemin (1), flosin B (2), stachyurin (3), casuarinin (4), casuariin (5), epipunicacortein A (6), and 2, 3-(S)-hexahydroxydiphenoyl-alpha/beta-D-glucose (7), together with one ellagic acid sulfate, 3-O-methyl-ellagic acid 4'-sulfate (8), ellagic acid (9), and four methyl ellagic acid derivatives, 3-O-methylellagic acid (10), 3,3'-di-O-methylellagic acid (3,8-Di-O-methylellagic acid,11), 3,4,3'-tri-O-methylellagic acid (12), and 3,4,8,9,10-pentahydroxydibenzo[b,d]pyran-6-one (13), were identified by the bioassay-directed isolation from the leaves of Lagerstroemia speciosa (L.) Pers. The chemical structures of these components were established on the basis of one- and two-dimensional NMR and high-resolution mass spectroscopic analyses. Other known compounds, including corosolic acid, gallic acid, 4-hydroxybenzoic acid, 3-O-methylprotocatechuic acid, caffeic acid, p-coumaric acid, kaempferol, quercetin, and isoquercitrin, were also isolated from the same plant. The obtained ellagitannins exhibited strong activities in both stimulating insulin-like glucose uptake (1-5 and 7) and inhibiting adipocyte differentiation (1 and 4) in 3T3-L1 cells. Meanwhile, ellagic acid derivatives (10-13) showed an inhibitory effect on glucose transport assay.
CONCLUSIONS:
This study is the first to report an inhibitory effect for methyl ellagic acid derivatives. | | Korean Society for Biotechnology and Bioengineering, 2011,4, 272-272. | | Chemical Constituents with DPPH Radical Scavenging, Elastase Inhnbition and Tyrosinase Inhibition Activities from Cleyera japonica Thunb[Reference: WebLink] | Bioassay-guided investigation of the stem of Cleyera japonica Thunb. led to the isolation of five compounds such as 3,5,7-trihydroxylchromone 3-O-α-L-rhamnopyranoside (1), aviculin (2), 3,3'-di-O-methylellagic acid (3,8-Di-O-methylellagic acid,3), 3,3'-di-O-methylellagic acid 4'-O-β-D-xylopyranoside (4) and betulinic acid (5). Their structures were elucidated on the basis of spectral studies as well as by comparison of their data with literature values.
METHODS AND RESULTS:
In order to study the skin related bioactivities for the isolated compounds, screenings on anti-oxidation, anti-tyrosinase and anti-elastase were conducted. For the anti-oxidation tests, compound 1, 2 and 3 showed strong DPPH radical scavenging activities with SC50 of 59.3, 58.1 and 123.3 μg/mL respectively, whose activities were comparable to a positive control BHT (SC50 89.0 μg/mL). On the tyrosinase inhibition studies, compound 4 (IC50 36.3 μg/mL) showed more potent activity than arbutin (IC50 67.2 μg/mL). On the elastase inhibition studies, compound 5 (IC50 17.9 μg/mL) showed higher activity than oleanolic acid (IC50 29.6 μg/mL), a positive control.
CONCLUSIONS:
Based on these results, C. japonica stem extracts could be potentially applicable as an ingredient in skin care formulations. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)