| Description: |
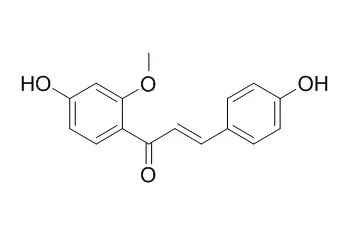
3-Deoxysappanchalcone is an effective HO-1 inducer at the translational level. 3-Deoxysappanchalcone has anti-inflammatory, and anti-influenza virus activities, the mechanism involved anti-apoptosis and anti-inflammation activities in vitro. It has inhibitory activity on MMP-9 expression and production in TNF-α-treated cells, is mediated by the suppression of AP-1 and NF-κB activation.
|
| Targets: |
HO-1 | mTOR | Akt | NO | IL Receptor | TNF-α | AP-1 | NF-kB | MMP(e.g.TIMP) |
| In vitro: |
| Planta Med. 2012 Jun;78(10):968-73. | | The protective effect of 3-deoxysappanchalcone on in vitro influenza virus-induced apoptosis and inflammation.[Pubmed: 22648377] | Influenza virus is one of the most important causes of acute respiratory disease. Viral infection and viral replication activate multiple cell signalling pathways. Apoptosis of infected cells and immune response against viral replication, which are generally considered to be protective mechanisms, are also probably mediated by viruses, which lead to severe health problems.
We previously reported that 3-Deoxysappanchalcone (3-DSC), a compound that is isolated from Caesalpinia sappan, exhibited in vitro anti-influenza activity.
METHODS AND RESULTS:
In the present study, we further identified that 3-DSC inhibited viral genomic replication and transcription only at a relatively high concentration. We then evaluated the effect of 3-DSC on the regulation of virus-induced cellular apoptosis. 3-DSC ameliorated virus-induced DNA fragmentation in a concentration-dependent manner, which tends to be a consequence of its inhibition of upstream caspase activation. 3-DSC also protected host cells against influenza-induced inflammation by suppressing CCL5 and CXCL10 secretions in endothelial cells and reducing the production of IL-6 and IL-1β in monocytes/macrophages.
CONCLUSIONS:
In conclusion, our results demonstrate that anti-influenza virus mechanisms of 3-DSC involved anti-apoptosis and anti-inflammation activities in vitro. Moreover, 3-DSC could be a promising drug candidate for influenza treatment. |
|
| In vivo: |
| Int Immunopharmacol . 2014 Oct;22(2):420-6. | | The anti-inflammatory effect of 3-deoxysappanchalcone is mediated by inducing heme oxygenase-1 via activating the AKT/mTOR pathway in murine macrophages[Pubmed: 25091623] | | Abstract
3-Deoxysappanchalcone (3-DSC), isolated from Caesalpinia sappan (Leguminosae), is a chalcone that exerts a variety of pharmacological activities. In the present study, we demonstrated that 3-DSC exerts anti-inflammatory activity in murine macrophages by inducing heme oxygenase-1 (HO-1) expression at the translational level. Treatment of RAW264.7 cells with 3-DSC induced HO-1 protein expression in a dose- and time-dependent manner without affecting HO-1 mRNA expression. Mitogen-activated protein kinase inhibitors or actinomycin D, a transcriptional inhibitor, did not block 3-DSC-mediated HO-1 induction. However, 3-DSC-mediated HO-1 induction was completely blocked by treatment with cycloheximide, a translational inhibitor, or rapamycin, an inhibitor of the mammalian target of rapamycin (mTOR). Strikingly, 3-DSC increased the phosphorylation level of mTOR downstream target molecules such as eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) and S6 kinase 1 (S6K1), as well as AKT in a dose- and time-dependent manner, suggesting that the 3-DSC induces HO-1 expression by activating the AKT/mTOR pathway. Consistent with the notion that HO-1 has anti-inflammatory properties, 3-DSC inhibited the production of nitric oxide (NO) and interleukin (IL)-6 in lipopolysaccharide (LPS)-stimulated RAW264.7 cells. Inhibition of HO-1 activity by treatment with tin protoporphyrin IX, a specific HO-1 inhibitor, abrogated the inhibitory effects of 3-DSC on the production of NO and IL-6 in LPS-stimulated RAW264.7 cells. Taken together, 3-DSC may be an effective HO-1 inducer at the translational level that has anti-inflammatory effects, and a valuable compound for modulating inflammatory conditions.
Keywords: 3-Deoxysappanchalcone; Anti-inflammatory effect; Heme oxygenase-1; Translation; mTOR. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)