| In vitro: |
| Phytochemistry, 1996, 41(3):775-778. | | Ellagic acid derivatives and naphthoquinones of Dionaea muscipula from in vitro cultures.[Reference: WebLink] |
METHODS AND RESULTS:
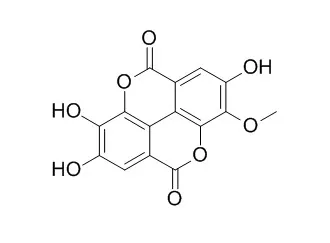
From Dionaea muscipula, obtained by in vitro culture, the known compounds plumbagin, chloroplumbagin and 8,8′-biplumbagin as naphthoquinones, 1-O-β-galloylglucose, ellagic acid,
3-O-methylellagic acid(3-Methyl ellagic acid), 3,3′-di-O-methylellagic acid and its 4-O-glucoside, and a new compound, the 4,4′-di-O-glucoside of 3,3′-di-O-methylellagic acid, were isolated.
CONCLUSIONS:
The assignments of NMR resonances of 3,3′-di-O-methylellagic acid 4-O-glucoside were substantiated by correlation techniques. | | Journal of Medicinal Plant Research (2011) 5(23) 5584-5589. | | Antioxidant and cytotoxic activity of the acetone extracts of root of Euphorbia hylonoma and its ellagic acid derivatives.[Reference: WebLink] |
METHODS AND RESULTS:
The acetone extracts and fractions prepared from the roots of Euphorbia hylonoma were investigated for 1, 1-diphenyl-2-pycrylhydrazyl (DPPH) free radical, superoxide anion and hydroxyl radical scavenging activity and cytotoxic activity against the human hepatoma cell line SMMC-7721, human cervix epitheloid carcinoma cell line HeLa and the human gastric cancer cell line SGC-7901. The acetone extract and fraction of EtOAc showed high antioxidant activities. The acetone extract and fraction of water showed a stronger cytotoxic effect on all tested cells, with IC50 values being less than 20 μg/mL. The acetone extract and fraction of water induced cell cycle arrest in G1 phase in SMMC-7721 cells.
CONCLUSIONS:
The major compounds isolated from the acetone extract were ellagic acid derivatives and showed weak cytotoxic activities on the tested cell lines, and these compound were 3,3',4-tri-O-methylellagic acid (1), 3,3'-di-O-methylellagic acid (2), 3-O-methylellagic acid (3-Methyl ellagic acid, 3) and 3,3'-di-O-methylellagic acid-4'-O-β-dxylopyranosid (4). Compound 3 showed better obvious radical scavenging activity than other tested compounds. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)