| Description: |
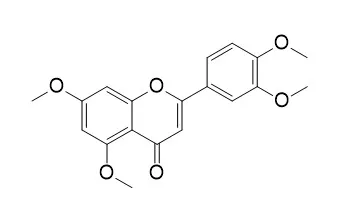
5,7,3',4'-Tetramethoxyflavone (TMF) possesses various bioactivities, including antifungal, antimalarial, antimycobacterial, and anti-inflammatory activities; it also exhibits chondroprotective activity by targeting β-catenin signaling in vivo and in vitro. TMF protects chondrocytes from ER stress-induced apoptosis through regulation of the IRE1α pathway.TMF inhibits the expression of tyrosinase, tyrosine-related protein (TRP)-1, and TRP-2 mRNA, which could be the mechanism of its melanogenesis inhibitory activity. |
| Targets: |
β-catenin | IRE1α | TRP-2 | TRP-1 | Antifection | TNF-α | PGE | PKA | cAMP | JNK | Caspase | Bcl/Bax | Antifection |
| In vitro: |
| Connect Tissue Res. 2018 Mar;59(2):157-166. | | 5,7,3',4'-Tetramethoxyflavone protects chondrocytes from ER stress-induced apoptosis through regulation of the IRE1α pathway.[Pubmed: 28436754 ] | To investigate the roles of endoplasmic reticulum (ER) transmembrane sensor inositol-requiring enzyme-1 (IRE1)α signaling in ER stress-induced chondrocyte apoptosis, and to determine the molecular mechanisms underlying chondroprotective activity of 5,7,3',4'-Tetramethoxyflavone (TMF) from Murraya exotica.
METHODS AND RESULTS:
IRE1α was knocked down by siRNA transfection in chondrocytes, which were harvested from rats' knee cartilages. Chondrocytes with IRE1α deficiency were administrated with tunicamycin (TM) and TMF. Chondrocyte apoptosis was quantified by flow cytometry and DAPI/TUNEL staining. Expression of mRNA and proteins was quantified by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and western-blot, respectively.
IRE1α deficiency significantly increased the rate of TM-induced chondrocyte apoptosis, down-regulated the expression of pro-survival factors XBP1S and Bcl-2, and up-regulated pro-apoptotic factors CHOP, p-JNK, and caspase-3. TMF suppressed TM-induced chondrocyte apoptosis by activating the expression of IRE1α, which reversed the expression patterns of downstream pro-survival and pro-apoptotic factors due to IRE1α deficiency.
CONCLUSIONS:
The mechanism of TMF in protecting chondrocytes against ER stress-induced apoptosis might be associated with regulating the activity of ER sensor IRE1α and its downstream pathway. | | J Nat Med. 2016 Apr;70(2):179-89. | | Simultaneous quantitative analysis of 12 methoxyflavones with melanogenesis inhibitory activity from the rhizomes of Kaempferia parviflora.[Pubmed: 26711832 ] | A methanol extract from the rhizomes of Kaempferia parviflora Wall. ex Baker (Zingiberaceae) has shown inhibitory effects against melanogenesis in theophylline-stimulated murine B16 melanoma 4A5 cells (IC50 = 9.6 μg/mL).
METHODS AND RESULTS:
Among 25 flavonoids and three acetophenones isolated previously (1-28), several constituents including 5-hydroxy-7,3',4'-trimethoxyflavone (6, IC50 = 8.8 μM), 5,7,3',4'-Tetramethoxyflavone (7, 8.6 μM), 5,3'-dihydroxy-3,7,4'-trimethoxyflavone (12, 2.9 μM), and 5-hydroxy-3,7,3',4'-tetramethoxyflavone (13, 3.5 μM) showed inhibitory effects without notable cytotoxicity at the effective concentrations. Compounds 6, 7, 12, and 13 inhibited the expression of tyrosinase, tyrosine-related protein (TRP)-1, and TRP-2 mRNA, which could be the mechanism of their melanogenesis inhibitory activity. In addition, a quantitative analytical method for 12 methoxyflavones (1, 2, 4-11, 13, and 14) in the extract was developed using HPLC. The optimal condition for separation and detection of these constituents were achieved on an ODS column (3 μm particle size, 2.1 mm i.d. × 100 mm) with MeOH-0.1 % aqueous acetic acid solvent systems as the mobile phase, and the detection and quantitation limits of the method were estimated to be 0.08-0.66 ng and 0.22-2.00ng, respectively. The relative standard deviation values of intra- and interday precision were lower than 0.95 and 1.08 %, respectively, overall mean recoveries of all flavonoids were 97.9-102.9 %, and the correlation coefficients of all the calibration curves showed good linearity within the test ranges. For validation of the protocol, extracts of three kinds of the plant's rhizomes collected from different regions in Thailand (Leoi, Phetchabun, and Chiang Mai provinces) were evaluated.
CONCLUSIONS:
The results indicated that the assay was reproducible, precise, and could be readily utilized for the quality evaluation of the plant materials. |
|
| In vivo: |
| J Agric Food Chem. 2012 Aug 22;60(33):8123-8. | | Identification of 5,7,3',4'-tetramethoxyflavone metabolites in rat urine by the isotope-labeling method and ultrahigh-performance liquid chromatography-electrospray ionization-mass spectrometry.[Pubmed: 22812915 ] | 5,7,3',4'-Tetramethoxyflavone (TMF), one of the major polymethoxyflavones (PMFs) isolated from Kaempferia parviflor , has been reported possessing various bioactivities, including antifungal, antimalarial, antimycobacterial, and anti-inflammatory activities. Although several studies on the TMF have been reported, the information about the metabolism of TMF and the structures of TMF metabolites is still not yet clear.
METHODS AND RESULTS:
In this study, an isotope-labeling method was developed for the identification of TMF metabolites. Three isotope-labeled TMFs (5,7,3',4'-tetramethoxy[3'-D(3)]flavone, 5,7,3',4'-tetramethoxy[4'-D(3)]flavone, and 5,7,3',4'-tetramethoxy[5,4'-D(6)]flavone) were synthesized and administered to rats. The urine samples were collected, and the main metabolites were monitored by ultrahigh-performance liquid chromatography-electrospray ionization-mass spectrometry.
CONCLUSIONS:
Five TMF metabolites were unambiguously identified as 3'-hydroxy-5,7,4'-trimethoxyflavone, 7-hydroxy-5,3',4'-trimethoxyflavone sulfate, 7-hydroxy-5,3',4'-trimethoxyflavone, 4'-hydroxy-5,7,3'-trimethoxyflavone, and 5-hydroxy-7,3',4'-trimethoxyflavone. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)