| Structure Identification: |
| Food Chem. 2011 Jul 15;127(2):394-403. | | Chemical composition and biological activity of Citrus jambhiri Lush.[Pubmed: 23140678 ] | The fresh peel of Citrus jambhiri was extracted with aqueous methanol and the residue was fractionated using light petroleum, chloroform and ethyl acetate.
METHODS AND RESULTS:
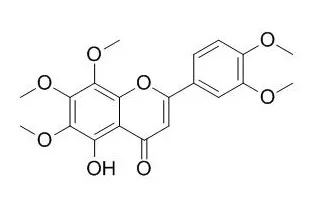
The constituents of the extracts were separated by column chromatography employing solvents of different polarity. The chemical structure of the isolated compounds was then identified by MS and NMR. Column chromatography of the petroleum fraction resulted in the isolation of nobiletin, 5-O-Demethylnobiletin, tangeretin, 5-hydroxy-3,6,7,8,3',4'-hexamethoxyflavone, 3,5,6,7,8,3',4'-heptamethoxyflavone, and a mixture of β-sitosterol and stigmasterol. The chloroform fraction afforded 6-demethoxynobiletin, 5,4'-dihydroxy-6,7,8,3'-tetramethoxyflavone, limonin and nomilin. The flavonoid glycosides naringin, hesperidin and neohesperidin were isolated from the ethyl acetate fraction. The chemical structure of the isolated compounds was established by MS and NMR (APT, COSY, HSQC, HMBC, and NOESY). LC-ESI-MS analysis of the ethyl acetate fraction afforded eight flavonoid glycosides, while the dichloromethane fraction of the defatted seeds contained seven limonoid aglycones. The chloroform fraction exerted the strongest DPPH(∗) free radical scavenging activity in comparison to other fractions.
CONCLUSIONS:
The petroleum fraction showed a significant inhibition of lipoxygenase indicating an anti-inflammatory action (IC(50) 29±1μg/mL). Some of the isolated polymethoxyflavones exhibited strong cytotoxicity against COS7, HeLa and Caco-2 cell lines. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)