| Description: |
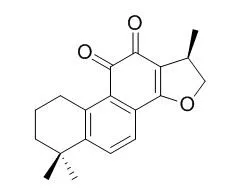
Cryptotanshinone is a potent STAT3 inhibitor with IC50 of 4.6 μM, and inhibits STAT3 Tyr705 phosphorylation in DU145 prostate cancer cells. It is also an AR inhibitor to suppress androgen/AR-mediated cell growth and PSA expression by blocking AR dimerization and the AR-coregulator complex formation. Cryptotanshinone has anti-atherosclerosis, neuroprotective, anti-cancer,and anti-neointimal formation activities.
Cryptotanshinone reverses DEX-induced androgen excess and ovarian IR in mice through activation of insulin signaling and the regulation of glucose transporters and hormone-synthesizing enzymes, it has an inhibitory effect on MMP-9 production and migration of human aortic smooth muscle cells treated with TNF-alpha in a dose-dependent manner.
|
| Targets: |
NF-kB | TNF-α | ROS | Caspase | p38MAPK | Akt | P450 (e.g. CYP17) | JNK | MMP(e.g.TIMP) | AP-1 | Androgen Receptor | ERK | PI3K | Bcl-2/Bax | COX |
| In vitro: |
| Apoptosis. 2011 Jul;16(7):696-707. | | Cryptotanshinone enhances TNF-α-induced apoptosis in chronic myeloid leukemia KBM-5 cells.[Pubmed: 21519916] | Cryptotanshinone is a biologically active compound from the root of Salvia miltiorrhiza.
In the present study, we investigated the molecular mechanisms by which Cryptotanshinone is in synergy with tumor necrosis factor-alpha (TNF-α) for the induction of apoptosis in human chronic myeloid leukemia (CML) KBM-5 cells.
METHODS AND RESULTS:
The co-treatment of Cryptotanshinone with TNF-α reduced the viability of the cells [combination index (CI) < 1]. Concomitantly, the co-treatment of Cryptotanshinone and TNF-α elicited apoptosis, manifested by enhanced the number of terminal deoxynucleotide transferase-mediated dUTP-nick-end labeling (TUNEL)-positive cells, the sub-G1 cell populations, and the activation of caspase-8 and -3, in comparison with the treatment with either drug alone. The treatment with Cryptotanshinone further suppressed TNF-α-mediated expression of c-FLIP(L), Bcl-x(L), but the increased level of tBid (a caspase-8 substrate). Furthermore, Cryptotanshinone activated p38 but not NF-κB in TNF-α-treated KBM-5 cells. The addition of a specific p38 MAPK inhibitor SB203580 significantly attenuated Cryptotanshinone/TNF-α-induced apoptosis. The combination treatment of Cryptotanshinone and TNF-α also stimulated the reactive oxygen species (ROS) generation. N-acetyl-L-cysteine (NAC, a ROS scavenger) was not only able to block Cryptotanshinone/TNF-α-induced ROS production but also the activation of caspase-8 and p38 MAPK.
CONCLUSIONS:
Overall, our findings suggest that Cryptotanshinone can sensitize TNF-α-induced apoptosis in human myeloid leukemia KBM-5 cells, which appears through ROS-dependent activation of caspase-8 and p38. | | Exp Brain Res. 2009 Feb;193(1):109-18. | | Cryptotanshinone protects primary rat cortical neurons from glutamate-induced neurotoxicity via the activation of the phosphatidylinositol 3-kinase/Akt signaling pathway.[Pubmed: 18936923] | Excitotoxicity contributes to neuronal death and is involved in the pathogenesis of neurodegenerative disorders such as Alzheimer's disease (AD).
In the present study, Cryptotanshinone, an active ingredient from a Chinese plant, Salvia miltiorrhiza, was investigated to assess its neuroprotective effects against glutamate-induced toxicity in primary culture of rat cortical neurons.
METHODS AND RESULTS:
Cryptotanshinone reversed glutamate-induced neuronal toxicity, which was characterized by decreased cell viability, increased lactate dehydrogenase release, neuronal DNA condensation, and the alteration of the expression of Bcl-2 family proteins. The neuroprotective effects of Cryptotanshinone could be blocked by LY294002 and wortmannin, two inhibitors of PI3K. The importance of the PI3K pathway was further confirmed by the activation of Akt and anti-apoptotic Bcl-2 by Cryptotanshinone in a PI3K-dependent manner.
CONCLUSIONS:
These results suggest that Cryptotanshinone protects primary cortical neurons from glutamate-induced neurotoxicity through the activation of PI3K/Akt pathway. Such neuroprotective effects may be of interest in AD and other neurodegenerative diseases. |
|
| In vivo: |
| Fertil Steril. 2014 Aug;102(2):589-596.e4. | | Cryptotanshinone reverses ovarian insulin resistance in mice through activation of insulin signaling and the regulation of glucose transporters and hormone synthesizing enzymes.[Pubmed: 24973798] | To investigate the effects of Cryptotanshinone (CRY), an active component of Chinese medicine, on ovarian androgen production, insulin resistance (IR), and glucose metabolism in mice.
METHODS AND RESULTS:
Animal model and in vitro tissue model.
University-affiliated laboratory.
Ovarian IR was induced by dexamethasone (DEX) in vivo. Animals were randomized to receive CRY treatment for 3 days or not. Ovulation rates, serum steroid levels, and glucose uptake in ovaries were quantified, and proteins in the phosphatidylinositol 3-hydroxy kinase pathway were measured. In vitro ovarian IR was also induced by DEX for 3 days. Ovarian steroid hormone secretion and glucose uptake were measured, and the hormone-synthesizing enzymes were determined by semiquantitative reverse transcription-polymerase chain reaction.
Ovarian glucose uptake, in vivo ovulation rate, serum and culture medium steroid level, and molecular expression of phosphatidylinositol 3-hydroxy kinase and steroidogenic enzymes.
Dexamethasone significantly increased ovulation rates in vivo and increased T and E2 production and decreased ovarian glucose uptake in vivo and in vitro. Cryptotanshinone significantly reduced ovulation rates in vivo and decreased T and estrogen production in vitro. Cryptotanshinone attenuated the inhibition of DEX on AKT2 and suppressed the up-regulation of CYP11 and CYP17 expression by DEX.
CONCLUSIONS:
Cryptotanshinone reversed DEX-induced androgen excess and ovarian IR in mice through activation of insulin signaling and the regulation of glucose transporters and hormone-synthesizing enzymes. This suggests a potential role for CRY in treating the ovulatory dysfunction associated with PCOS. | | Leuk Lymphoma . 2015 Mar;56(3):730-8. | | Cryptotanshinone acts synergistically with imatinib to induce apoptosis of human chronic myeloid leukemia cells[Pubmed: 24884318] | | Abstract
Imatinib resistance has emerged as a significant clinical problem in chronic myeloid leukemia (CML) treatment. In this study, we investigated the effect and mechanism of combination treatment with imatinib and Cryptotanshinone (CPT) in CML cells. Cotreatment with imatinib and CPT showed a significant synergistic killing effect in both imatinib sensitive and resistant CML cell lines, as well as primary CML cells. Furthermore, combination treatment induced apoptosis significantly, as indicated by increases in apoptotic cell fraction and activities of proapoptotic proteins. Subsequent studies revealed that CPT significantly inhibited Bcr/Abl protein expression, as well as phosphorylation expression levels of signal transducer and activator of transcription 3 (STAT3), mammalian target of rapamycin (mTOR) and eukaryotic translation initiation factor 4E (eIF4E), which are critical mediators of Bcr/Abl transformation. Furthermore, CPT in combination with imatinib dramatically decreased the activity of the Bcr/Abl pathway in both K562 and K562-R cells. Our results demonstrated that CPT increased imatinib-induced apoptosis in a Bcr/Abl dependent manner, suggesting a novel strategy for the treatment of CML.
Keywords: Bcr/Abl pathway; CPT; imatinib resistance; synergism. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)