| Description: |
6-Gingerol possesses anti-adipogenic, anti-tumorigenic, anti-invasive, antioxidant, anti-inflammatory, and pro-apoptotic activities, it stimulates apoptosis through upregulation of NAG-1 and G1 cell cycle arrest through downregulation of cyclin D1, multiple mechanisms appear to be involved in 6-gingerol action, including protein degradation as well as β-catenin, PKCε, and GSK-3β pathways. 6-Gingerol can effectively suppress adipogenesis and that it exerts its role mainly through the significant down-regulation of PPARγ and C/EBPα and subsequently inhibits FAS and aP2 expression, also inhibit differentiation in 3T3-L1 cells by attenuating the Akt/GSK3β pathway. |
| In vitro: |
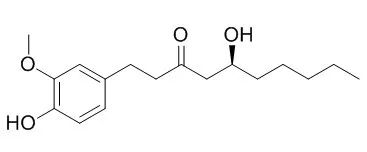
| Phytomedicine. 2013 Apr 15;20(6):481-7. | | 6-gingerol prevents adipogenesis and the accumulation of cytoplasmic lipid droplets in 3T3-L1 cells.[Pubmed: 23369342 ] | 6-Gingerol ((S)-5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-3-decanone) is one of the pungent constituents of Zingiber zerumbet (L) Smith (Zingiberaceae family).
METHODS AND RESULTS:
In this study, we investigated the effects of 6-Gingerol on the inhibition of adipogenesis in 3T3-L1 cells. After treatment with 6-Gingerol in differentiation medium for 4 or 8 days, the 3T3-L1 cells were lysed for experimental analysis. Cells were stained with Oil-Red-O to detect oil droplets in adipocytes. The 3T3-L1 cells were lysed and measured for triglyceride contents. The protein expression of adipogenesis-related transcription factor was evaluated by Western blot analysis. 6-Gingerol suppressed oil droplet accumulation and reduced the droplet size in a concentration (5-15 μg/ml)- and time-dependent manner. Treatment of 3T3-L1 cells with 6-Gingerol reduced the protein levels of peroxisome proliferator-activated receptor (PPAR)γ and CCAAT/enhancer-binding protein (C/EBP)α. Additionally, the protein levels of fatty acid synthase (FAS) and adipocyte-specific fatty acid binding protein (aP2) decreased upon treatment with 6-Gingerol. Meanwhile, 6-Gingerol diminished the insulin-stimulated serine phosphorylation of Akt (Ser473) and GSK3β (Ser9).
CONCLUSIONS:
These results suggest that 6-Gingerol effectively suppresses adipogenesis and that it exerts its role mainly through the significant down-regulation of PPARγ and C/EBPα and subsequently inhibits FAS and aP2 expression. 6-Gingerol also inhibited differentiation in 3T3-L1 cells by attenuating the Akt/GSK3β pathway. Our findings provide important insights into the mechanisms underlying the anti-adipogenic activity of 6-Gingerol. | | Mol Nutr Food Res. 2010 Nov;54(11):1618-27. | | Anti-invasion effects of 6-shogaol and 6-gingerol, two active components in ginger, on human hepatocarcinoma cells.[Pubmed: 20521273] | Hepatocellular carcinoma is the most common type of liver cancer and is highly metastatic. Metastasis is considered to be the major cause of death in cancer patients. Ginger is a natural dietary rhizome with anti-oxidative, anti-inflammatory, and anti-carcinogenic activities. The aims of this study were to evaluate the anti-invasion activity of 6-shogaol and 6-Gingerol, two compounds found in ginger, on hepatoma cells.
METHODS AND RESULTS:
The migratory and invasive abilities of phorbol 12-myristate 13-acetate (PMA)-treated HepG2 and PMA-untreated Hep3B cells were both reduced in a dose-dependent manner by treatment with 6-shogaol and 6-Gingerol. Upon incubation of PMA-treated HepG2 cells and PMA-untreated Hep3B cells with 6-shogaol and 6-Gingerol, matrix metalloproteinase (MMP)-9 activity decreased, whereas the expression of tissue inhibitor metalloproteinase protein (TIMP)-1 increased in both cell types. Additionally, urokinase-type plasminogen activator activity was dose-dependently decreased in Hep3B cells after incubation with 6-shogaol for 24 h. Analysis with semi-quantitative reverse transcription-PCR showed that the regulation of MMP-9 by 6-shogaol and 6-Gingerol and the regulation of TIMP-1 by 6-shogaol in Hep3B cells may on the transcriptional level.
CONCLUSIONS:
These results suggest that 6-shogaol and 6-Gingerol might both exert anti-invasive activity against hepatoma cells through regulation of MMP-9 and TIMP-1 and that 6-shogaol could further regulate urokinase-type plasminogen activity. | | Chem Biol Interact. 2010 Apr 15;185(1):12-7. | | Genotoxic effect of 6-gingerol on human hepatoma G2 cells.[Pubmed: 20167213 ] | 6-Gingerol, a major component of ginger, has antioxidant, anti-apoptotic, and anti-inflammatory activities. However, some dietary phytochemicals possess pro-oxidant effects as well, and the risk of adverse effects is increased by raising the use of doses. The aim of this study was to assess the genotoxic effects of 6-Gingerol and to clarify the mechanisms, using human hepatoma G2 (HepG2) cells.
METHODS AND RESULTS:
Exposure of the cells to 6-Gingerol caused significant increase of DNA migration in comet assay, increase of micronuclei frequencies at high concentrations at 20-80 and 20-40 microM, respectively. These results indicate that 6-Gingerol caused DNA strand breaks and chromosome damage. To further elucidate the underlying mechanisms, we tested lysosomal membrane stability, mitochondrial membrane potential, the intracellular generation of reactive oxygen species (ROS) and reduced glutathione (GSH). In addition, the level of oxidative DNA damage was evaluated by immunocytochemical analysis on 8-hydroxydeoxyguanosine (8-OHdG). Results showed that lysosomal membrane stability was reduced after treatment by 6-Gingerol (20-80 microM) for 40 min, mitochondrial membrane potential decreased after treatment for 50 min, GSH and ROS levels were significantly increased after treatment for 60 min.
CONCLUSIONS:
These suggest 6-Gingerol induces genotoxicity probably by oxidative stress; lysosomal and mitochondrial damage were observed in 6-Gingerol-induced toxicity. | | PLoS One . 2014 Aug 26;9(8):e104401. | | [6]-Gingerol induces caspase-dependent apoptosis and prevents PMA-induced proliferation in colon cancer cells by inhibiting MAPK/AP-1 signaling[Pubmed: 25157570] | | We report mechanism-based evidence for the anticancer and chemopreventive efficacy of [6]-gingerol, the major active principle of the medicinal plant, Ginger (Zingiber officinale), in colon cancer cells. The compound was evaluated in two human colon cancer cell lines for its cytotoxic effect and the most sensitive cell line, SW-480, was selected for the mechanistic evaluation of its anticancer and chemopreventive efficacy. The non-toxic nature of [6]-gingerol was confirmed by viability assays on rapidly dividing normal mouse colon cells. [6]-gingerol inhibited cell proliferation and induced apoptosis as evidenced by externalization of phosphatidyl serine in SW-480, while the normal colon cells were unaffected. Sensitivity to [6]-gingerol in SW-480 cells was associated with activation of caspases 8, 9, 3 &7 and cleavage of PARP, which attests induction of apoptotic cell death. Mechanistically, [6]-gingerol down-regulated Phorbol Myristate Acetate (PMA) induced phosphorylation of ERK1/2 and JNK MAP kinases and activation of AP-1 transcription factor, but had only little effects on phosphorylation of p38 MAP kinase and activation of NF-kappa B. Additionally, it complemented the inhibitors of either ERK1/2 or JNK MAP kinase in bringing down the PMA-induced cell proliferation in SW-480 cells. We report the inhibition of ERK1/2/JNK/AP-1 pathway as a possible mechanism behind the anticancer as well as chemopreventive efficacy of [6]-gingerol against colon cancer. | | Food Funct . 2015 Oct;6(10):3334-41. | | 6-Gingerol modulates proinflammatory responses in dextran sodium sulfate (DSS)-treated Caco-2 cells and experimental colitis in mice through adenosine monophosphate-activated protein kinase (AMPK) activation[Pubmed: 26263169] | | Background: 6-Gingerol has been reported to have anti-inflammatory effects in different experimental settings. The present study aimed at evaluating the effect of 6-Gingerol on dextran sodium sulfate (DSS)-induced barrier impairment and inflammation in vitro and in vivo.
Methods: a differentiated Caco-2 monolayer was exposed to DSS and treated with different concentrations of 6-Gingerol (0, 1, 5, 10, 50, and 100 μM). Changes in intestinal barrier function were determined using transepithelial electrical resistance (TEER). The anti-inflammatory activity of 6-Gingerol was examined as changes in the expression of proinflammatory cytokine using quantitative real-time PCR. Western blotting was employed to determine the activation of adenosine monophosphate-activated protein kinase (AMPK). Mice with DSS-induced colitis were given different oral dosages of 6-Gingerol daily for 14 days. Body weight and colon inflammation were evaluated, and level of proinflammatory cytokines in colon tissues was measured.
Results: 6-Gingerol treatment was shown to restore impaired intestinal barrier function and to suppress proinflammatory responses in DSS-treated Caco-2 monolayers. We found that AMPK was activated on 6-Gingerol treatment in vitro. In animal studies, 6-Gingerol significantly ameliorated DSS-induced colitis by restoration of body weight loss, reduction in intestinal bleeding, and prevention of colon length shortening. In addition, 6-Gingerol suppressed DSS-elevated production of proinflammatory cytokines (IL-1β, TNFα, and IL-12).
Conclusion: our findings highlight the protective effects of 6-Gingerol against DSS-induced colitis. We concluded that 6-Gingerol exerts anti-inflammatory effects through AMPK activation. It is suggested that 6-Gingerol has a promising role in treatment of IBD. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)