| Structure Identification: |
| Zhong Yao Cai. 2015 May;38(5):988-91. | | Chemical Constituents from Processed Products of Aconitum Vilmoriniani Radix.[Pubmed: 26767293] | To investigate the chemical constituents of the processed products of Aconitum Vilmorinian Radix.
METHODS AND RESULTS:
The constituents were isolated by repeated column chromatography over silica gel, alumina and RP-C18 as well as recrystallization. The structures were elucidated on the basis of spectral analysis and physicochemical properties.
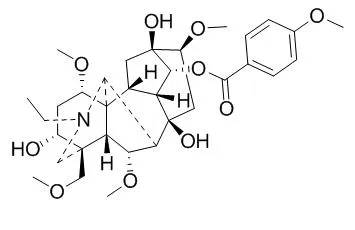
Ten compounds were obtained from the methanol extract, and they were identified as yunaconitine (1), 8-deacetyl-yunaconitine (2), geniculatine C (3), vilmorrianine B (4), vilmorrianine C(5), vilmorrianine D (6), talatisamine (7), β-sitosterol (8), β-daucosterol (9) and β-sitosterol acetate (10).
CONCLUSIONS:
All compounds are obtained from the processed products of Aconitum Vilmoriniani Radix for the first time. | | J Sep Sci. 2013 Aug;36(16):2680-5. | | Accelerated solvent extraction and pH-zone-refining counter-current chromatographic purification of yunaconitine and 8-deacetylyunaconitine from Aconitum vilmorinianum Kom.[Pubmed: 23784883] |
METHODS AND RESULTS:
This study aimed to seek an efficient method to extract and purify yunaconitine and 8-Deacetylyunaconitine from Aconitum vilmorinianum Kom. by accelerated solvent extraction combined with pH-zone-refining counter-current chromatography.
The major extraction parameters for accelerated solvent extraction were optimized by an orthogonal test design L9 (3)(4). Then a separation and purification method was established using pH-zone-refining counter-current chromatography with a two-phase solvent system composed of petroleum ether/ethyl acetate/methanol/water (5:5:2:8, v/v) with 10 mM triethylamine in the upper phase and 10 mM HCl in the lower phase. From 2 g crude extract, 224 mg of 8-Deacetylyunaconitine (I) and 841 mg of yunaconitine (II) were obtained with a purity of over 98.0%.
CONCLUSIONS:
The chemical structures were identified by ESI-MS and (1)H and (13)C NMR spectroscopy. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)