| Description: |
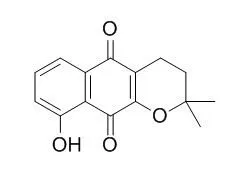
9-Hydroxy-alpha-lapachone can highly inhibit the growth of H. pylori, it exhibits potent inhibitory activity against H. pylori Cystathionine gamma-synthase, with IC(50) values of 4.64 microM. It may have anti-inflammatory activity, it exhibits potent inhibitory effects on lipopolysaccharide- induced NO synthesis in RAW 264.7 cells, with IC50 values of 4.64 microM. |
| Targets: |
Antifection | NO |
| In vitro: |
| Arch Pharm Res. 2010 Mar;33(3):381-5. | | Naphthoquinones from Catalpa ovata and their inhibitory effects on the production of nitric oxide.[Pubmed: 20361302] |
METHODS AND RESULTS:
Bioassay-guided fractionation of a CH2Cl2-soluble fraction of the stems of Catalpa ovata led to isolation of a new naphthoquinone, 4-hydroxy-2-(2-methoxy-3-hydroxy-3-methyl-but-1-enyl)-4-hydro-1H-naphthalen-1-one (10), together with nine known compounds, catalponol (1), catalponone (2), catalpalactone (3), alpha-lapachone (4), 9-Hydroxy-alpha-lapachone (5), 4,9-dihydroxy-alpha-lapachone (6), 9-methoxy-alpha-lapachone (7), 4-oxo-alpha-lapachone (8), and 9-methoxy-4-oxo-alpha-lapachone (9). The structures were elucidated on the basis of spectroscopic analyses. The inhibitory effects of these isolates on lipopolysaccharide-induced NO synthesis in RAW 264.7 cells were evaluated.
CONCLUSIONS:
Among them, catapalactone (3), 9-Hydroxy-alpha-lapachone (5) and 4,9-dihydroxy-alpha-lapachone (6) exhibited potent inhibitory effects, with IC(50) values of 9.80, 4.64 and 2.73 microM, respectively. |
|
| In vivo: |
| J Biochem. 2008 Jan;143(1):59-68. | | Enzymatic characterization and inhibitor discovery of a new cystathionine {gamma}-synthase from Helicobacter pylori.[Pubmed: 17981822] | Cystathionine gamma-synthase (CGS) catalyses the first step of the transsulfuration pathway that converts l-cysteine to l-homocysteine in bacteria, whereas this pathway is absent in human.
METHODS AND RESULTS:
In this report, we identified a new metB gene from Helicobacter pylori strain SS1, and the recombinant H. pylori Cystathionine gamma-synthase (HpCGS) was successfully cloned, expressed and purified in Escherichia coli system. Enzymatic study of HpCGS indicated that the K(m) and k(cat)/K(m) values against the substrate O-succinyl-l-homoserine (l-OSHS) were 3.02 mM and 98.7 M(-)(1)s(-)(1), respectively. Moreover, four natural products (alpha-lapachone, 9-Hydroxy-alpha-lapachone, Paulownin and Yangambin, Fig. 1) were discovered to demonstrate inhibitory activities against HpCGS with IC(50) values of 11 +/- 3, 9 +/- 1, 19 +/- 2 and 27 +/- 6 microM, respectively.
CONCLUSIONS:
All these four inhibitors prevent the binding of l-OSHS to HpCGS in a non-competitive fashion. In vitro antibacterial assays further indicated that these four discovered compounds could highly inhibit the growth of H. pylori and exhibited strong inhibitory specificity against H. pylori related to E. coli. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)