| Description: |
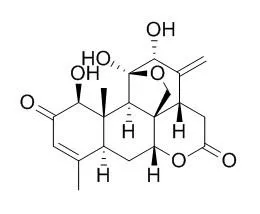
Ailanthone has anti-inflammatory, anti-HIV, anti-malarial, anti-allergic, antiulcer and antimicrobial activities; it also has potent antineoplastic activity against hepatocellular carcinoma (HCC), it can inhibit huh7 cancer cell growth via cell cycle arrest and apoptosis in vitro and in vivo. Ailanthone has significant pre-emergence herbicide activity , is directly correlated to Ailanthone concentration. |
| Targets: |
HIV | Antifection |
| In vitro: |
| Plant & Soil, 2003, 256(1):85-99. | | Herbicidal effects under field conditions of Ailanthus altissima bark extract, which contains ailanthone.[Reference: WebLink] |
METHODS AND RESULTS:
These application rates provided herbicidal activity equivalent to 4.5, 2.2, 1.1, 0.6, 0.3, and 0.0 kg of pure Ailanthone per hectare, based on the results of a laboratory bioassay of extract and pure Ailanthone.
Strong herbicidal effects were observed within several days.
Even the lowest rate caused mortality and injury in excess of 50% for nine of the 17 species, and a significant reduction in shoot biomass for 13 species. The second field trial tested the ability of bark extract to control weeds under field conditions with horticultural crops (bush bean, cauliflower, sweet corn, tomato). A. altissima bark extract was sprayed post-emergence at rates of 99, 50, 26, 13, and 0 kg ha–1, providing herbicidal activity equivalent to 1.1, 0.6, 0.3, 0.14, and 0.0 kg of pure Ailanthone per hectare. Extract treatment provided partial weed control (greatest reduction in weed biomass was 40%), but also caused serious crop injury. Bush bean was the only crop that showed a significant increase in shoot biomass and fruit yield, compared to the non-weeded control.
CONCLUSIONS:
None of the crops, regardless of application rate, showed a level of shoot biomass or fruit yield comparable to the hand-weeded control.
The herbicidal effects of A. altissima bark extract declined within the first few weeks after application, supporting previous evidence that Ailanthone is rapidly degraded under field conditions. |
|
| In vivo: |
| Anticancer Res. 1988 Jul-Aug;8(4):573-9. | | Antitumor activity of novel ailanthone derivatives in vitro and in vivo.[Pubmed: 3178149] | The antitumor activities of two Ailanthone derivatives with 15 beta-acyloxy side chains were investigated.
METHODS AND RESULTS:
The cytotoxic activity of 11 beta, 20-epoxy-1 beta, 11 alpha, 12 alpha-trihydroxy-15-beta-[E)-3-methyl-2-octenoyl) oxypicras-3,13(21)-diene-2,16-dione (SUN2071) and 11 beta, 20-epoxy-1 beta, 11 alpha, 12 alpha-trihydroxy-15 beta-[E)-2-undecenoyl) oxypicras-3,13(21)-diene-2,16-dione (SUN0237) was close to that of bruceantin and vincristine.
SUN2071 was shown to be a potent inhibitor of protein synthesis in L1210 cultured cells.
When administered i.p. to i.p. inoculated P388 leukemia mice, daily treatment with SUN2071 and SUN0237 significantly increased the lifespan (increases in lifespan in excess of 100% were achieved). These increases were comparable to those achieved with vincristine. The therapeutic ratio of SUN2071 was also close to that of vincristine. However, the compounds were ineffective when administered as a single injection. Daily i.p. treatment with SUN2071 demonstrated significant tumor growth inhibition in mice inoculated s.c. with colon-38 and moderate activity against i.p. L1210 leukemia and i.p. B16 melanoma. The compounds were ineffective when tested against the Lewis lung carcinoma and colon-26.
CONCLUSIONS:
In a preliminary toxicological study, SUN2071 at a therapeutic dose in daily consecutive i.p. injection produced leucopenia. | | Farmaco Sci. 1981 Feb;36(2):116-22. | | Antiamebic properties of some derivatives of ailantone and quassin.[Pubmed: 7227501] | | Owing to its high toxicity, Ailanthone, one of the most potent in vivo amoebicidal drugs of natural origin, cannot be safely employed in clinical trials. With the aim of obtaining a compound with a better therapeutic index and of studying possible relationships between biological activity and chemical structure, many derivatives of Ailanthone and of the chemically related, although biologically inactive, quassin have been prepared and tested. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)