| In vitro: |
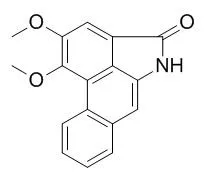
| Planta Med. 2004 May;70(5):391-6. | | Aristolactam BII of Saururus chinensis attenuates glutamate-induced neurotoxicity in rat cortical cultures probably by inhibiting nitric oxide production.[Pubmed: 15124081] | Saurolactam and Aristolactam BII, aristolactam-type alkaloids isolated from the aerial part of Saururus chinensis (Lour.) Ball (Saururaceae), showed significant neuroprotective activity against glutamate-induced toxicity in primary cultured rat cortical cells.
METHODS AND RESULTS:
The action mechanism of Aristolactam BII, the more potent neuroprotective compound, was investigated using primary cultures of rat cortical cells as an in vitro system. Aristolactam BII attenuated glutamate-induced neurotoxicity significantly when it was added immediately or up to 9 h after the excitotoxic glutamate challenge. The alkaloid could not protect cultured neuronal cells from neurotoxicity induced by kainic acid or N-methyl- D-aspartate in a pre-treatment paradigm. However, Aristolactam BII successfully reduced the overproduction of nitric oxide and the level of cellular peroxide in cultured neurons when it was treated as a post-treatment paradigm.
CONCLUSIONS:
These results may suggest that Aristolactam BII exerts its significant neuroprotective effects on glutamate-injured primary cultures of rat cortical cells by directly inhibiting the production of nitric oxide. | | Nat Prod Commun. 2010 Feb;5(2):253-8. | | Aristolactams, 1-(2-C-methyl-beta-D-ribofuranosyl)-uracil and other bioactive constituents of Toussaintia orientalis.[Pubmed: 20334138] |
METHODS AND RESULTS:
The new aristolactam alkaloid toussalactam {2-hydroxy-1,6-dimethoxy-5H-dibenzo[cdf]indol-4-one} and the known ones, namely aristolactam AII, Aristolactam BII, piperolactam C and aristolactam FII; 1-(2-C-methyl-beta-D-ribofuranosyl)-uracil, 3,4,5-trimethoxyphenyl-beta-D-glucopyranoside, and three catechinoids were isolated from the cytotoxic Toussaintia orientalis Verdc stem and root bark extracts, and their structures established based on analysis of spectroscopic data.
CONCLUSIONS:
The aristolactams exhibited antimicrobial and antiinflammatory activity, aristolactam FII showing almost the same level of activity as the standard anti-inflammatory agent Indomethacin.
The compounds also exhibited either mild or no antiproliferative and cytotoxic activities, except aristolactam FII that showed the same level of cytotoxicity as the standard drug Camptothecin. 1-(2-C-Methyl-beta-D-ribofuranosyl)-uracil, which is being reported for the first time as a natural product, was inactive in the antibacterial, antifungal, antiinflammatory, antiproliferative and cytotoxicity assays. | | J. Appl. Pharm. Sci., 2014, 4(5):87-91. | | Antioxidant and Anti-tyrosinase Activities from Piper officinarum C.DC (Piperaceae).[Reference: WebLink] | The present study was carried out to evaluate antioxidant and anti-tyrosinase activities from Piper officinarum stems, as well as investigation of its chemical constituents.
METHODS AND RESULTS:
In a series of in vitro assays, antioxidant (DPPH radical scavenging and total phenolic content) and anti-tyrosinase (mushroom tyrosinase) activities of various extracts of stem were evaluated. The isolation and purification of the constituents were carried out on the extracts using various chromatographic methods and identified by direct comparison of their spectroscopic data with respective published data. The results showed that the methanol extracts showed the highest DPPH (80.0%) at 1 mg/ml, as well as total phenolic content (50.5%). Phytochemical analysis of the stem extracts have isolated five compounds identified as 4-allyl resorcinol (1), aristolactam AII (2) Aristolactam BII (3), stigmast-4-en-3-one (4) and 6-hydroxystigmast-4-en-3-one (5). Compound (1), (2) and (3) showed significant activity towards DPPH radical scavenging (I%=17.3-28.1%), while (2), (3) and (4) demonstrated potent inhibitory effects against tyrosinase mushroom (I%=11.1-24.4%).
CONCLUSIONS:
The results showed that the stem extracts has significant antioxidant activity that may help to discover new chemical classes of natural antioxidant substances. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)