| In vitro: |
| Bioorg Med Chem Lett. 2009 Jun 1;19(11):3036-40. | | Synthesis of aristolactam analogues and evaluation of their antitumor activity.[Pubmed: 19394218] | A series of natural aristolactams and their analogues have been prepared and evaluated for antitumor activity against human cancer cells, including multi-drug resistant cell lines.
METHODS AND RESULTS:
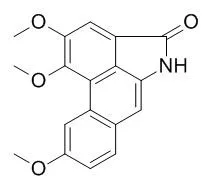
Naturally occurring aristolactams, such as aristolactam BII (cepharanone B), Aristolactam BIII, aristolactam FI (piperolactam A), N-methyl piperolactam A, and sauristolactam showed moderate antitumor activities in selected cell lines.
However, several synthetic aristolactam derivatives exhibited potent antitumor activities against a broad array of cancer cell lines with GI(50) values in the submicromolar range. | | Planta Med. 2005 Jun;71(6):535-42. | | New cytotoxic cyclobutanoid amides, a new furanoid lignan and anti-platelet aggregation constituents from Piper arborescens.[Pubmed: 15971125] |
METHODS AND RESULTS:
Three new cyclobutanoid amides with trans-trans-trans configurations, piperarborenine C, piperarborenine D and piperarborenine E, and a new furanoid lignan, (+)-arborone, together with twelve known compounds, were isolated from the stems of Piper arborescens. The structures of these new compounds were determined by means of spectral analyses. Piperarborenine C, (+)-diayangambin, piplartine, piperolactam B, piperolactam C, Aristolactam BIII, goniothalactam, and methyl trans-3,4,5-trimethoxycinnamate possessed anti-platelet aggregation activity in vitro. Among them, piplartine showed the most potent anti-platelet aggregation activity induced by collagen and showed an IC50 value of 21.5 microM.
CONCLUSIONS:
Piperarborenines A - E, piperarborenine, aristololactam BIII and goniothalactam showed significant cytotoxic activity (IC50 values < 4 microg/mL) against P-388, HT-29 and A549 cell lines in vitro. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)