| Kinase Assay: |
| J Org Chem. 2014 Dec 5;79(23):11722-8. | | Synthesis of multibranched australine derivatives from reducing castanospermine analogues through the Amadori rearrangement of gem-diamine intermediates: selective inhibitors of β-glucosidase.[Pubmed: 25390345] |
METHODS AND RESULTS:
A practical one-pot synthesis of bi- and triantennated Australine analogues from a pivotal sp(2)-iminosugar-type reducing castanospermine precursor is reported. The transformation involves a gem-diamine intermediate that undergoes the indolizidine → pyrrolizidine Amadori-type rearrangement and proceeds under strict control of the generalized anomeric effect to afford a single diastereomer.
CONCLUSIONS:
The final compounds behave as selective competitive inhibitors of β-glucosidase and are promising candidates as pharmacological chaperones for Gaucher disease. | | Biochemistry. 1989 Mar 7;28(5):2027-34. | | Australine, a pyrrolizidine alkaloid that inhibits amyloglucosidase and glycoprotein processing.[Pubmed: 2497772] |
METHODS AND RESULTS:
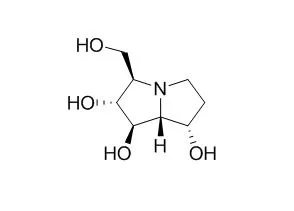
Australine [(1R,2R,3R,7S,7aR)-3-(hydroxymethyl)-1,2,7-trihydroxypyrrolizid ine] is a polyhydroxylated pyrrolizidine alkaloid that was isolated from the seeds of the Australian tree Castanospermum australe and characterized by NMR and X-ray diffraction analysis [Molyneux et al. (1988) J. Nat. Prod. (in press)]. Since swainsonine and catanospermine are polyhydroxylated indolizidine alkaloids that inhibit specific glycosidases, we tested Australine against a variety of exoglycosidases to determine whether it would inhibit any of these enzymes.Australine also inhibited the glycoprotein processing enzyme glucosidase I, but had only slight activity toward glucosidase II.
CONCLUSIONS:
When incubated with cultured cells, this alkaloid inhibited glycoprotein processing at the glucosidase I step and caused the accumulation of glycoproteins with Glc3Man7-9(GlcNAc)2-oligosaccharides. |
|
| Structure Identification: |
| Org Lett. 2015 Feb 6;17(3):716-9. | | Synthesis and glycosidase inhibition of australine and its fluorinated derivatives.[Pubmed: 25621897] |
METHODS AND RESULTS:
Australine (1), 7-epi-Australine (2), and their C-7-fluorinated derivatives 4 and 5 have been synthesized efficiently from D-arabinose-derived cyclic nitrone 11. Fluorination at the C-7 position enhanced the inhibition against A. niger α-glucosidase, and this constitutes the first example of fluorination substitution for a hydroxyl increasing the inhibition of any glycosidases.
CONCLUSIONS:
The enantiomers synthesized from nitrone ent-11 showed no inhibition of the corresponding enzymes. | | Chemistry. 2013 Aug 5;19(32):10595-604. | | Stereocomplementary routes to hydroxylated nitrogen heterocycles: total syntheses of casuarine, australine, and 7-epi-australine.[Pubmed: 23828462] |
METHODS AND RESULTS:
Addition of lithiated 1-benzyloxyallene to a D-arabinose-derived cyclic nitrone occurred with perfect diastereoselectivity furnishing a bicyclic 1,2-oxazine derivative, which is an excellent precursor for pyrrolizidine alkaloids hydroxylated at C-7 with optional configuration at this stereogenic center. Depending on the stage of the N-O bond cleavage and ring re-closure, 7-hydroxypyrrolizidines with 7R or 7S configuration were obtained, as a result of completely selective addition reactions occurring complementarily at the bottom or top face of the endocyclic C-C double bond in six- and five-membered B rings, respectively.
CONCLUSIONS:
Applicability of these stereodivergent routes to obtain polyhydroxy pyrrolizidine alkaloids is demonstrated by the efficient syntheses of casuarine and Australine as examples of the two classes of diversely configured 7-hydroxypyrrolizidine alkaloids. An alternative synthesis of Australine and two strategies for the preparation of 7-epi-Australine are also reported, which demonstrate that the stereoselectivity of hydride reduction of an exocyclic C-O double bond is independent of the ring size, occurring preferentially from the top face either in a six- or five-membered ring. | | J Org Chem. 2010 Feb 5;75(3):815-24. | | Total synthesis of uniflorine A, casuarine, australine, 3-epi-australine, and 3,7-di-epi-australine from a common precursor.[Pubmed: 20028000] |
METHODS AND RESULTS:
A flexible method for the diastereoselective total synthesis of the pyrrolizidine alkaloids uniflorine A, casuarine, Australine, and 3-epi-Australine and the unnatural epimer 3,7-di-epi-Australine from a common chiral 2,5-dihydropyrrole precursor is described. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)