| Description: |
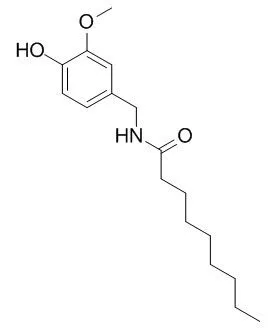
Nonivamide, present in chili peppers, is commonly manufactured synthetically and used as a food additive to add pungency to seasonings, flavorings, and spice blends.
Nonivamide and capsaicin are novel skin permeation enhancers for indometacin.
Nonivamide has antioxidant, anti-obesity, and anti-inflammatory effects, it reduces energy intake, enhances energy metabolism, decreases serum triacylglycerol content, and inhibits adipogenesis via activation of the transient receptor potential cation channel subfamily V member 1 (TRPV1). |
| In vitro: |
| Chem Biol Interact. 2009 Jul 15;180(2):183-92. | | Protective effect and relation structure-activity of nonivamide and iododerivatives in several models of lipid oxidation.[Pubmed: 19497416] | The introduction of an iodine atom on the vanillyl moiety of Nonivamide causes a switch in the vanilloid activity (TRPV1 antagonism versus TRPV1 desensitization) and nullifies the aversive properties of capsaicinoids. In the present study we investigated the effect of iodination in the vanillyl moiety on the antioxidant activity of capsaicinoids.
METHODS AND RESULTS:
To this aim, we have compared the protective effects of Nonivamide and three iododerivatives, 2-iodoNonivamide (2IN), 5-iodoNonivamide (5IN), and 6-iodoNonivamide (6IN) in a series of in vitro models of lipid oxidation, namely the autoxidation and the FeCl(3)-mediated oxidation of linoleic acid at 37 degrees C and the thermal (140 degrees C), solvent-free oxidation of cholesterol. All tested compounds could protect linoleic acid and cholesterol against oxidative degradation, the order of potency being: Nonivamide>5IN>6IN approximately 2IN. Our results show that, in general, the introduction of an iodine atom on the vanillyl moiety of Nonivamide causes a decrease in the antioxidant activity, and this effect is sensitive to the position of iodine on the aromatic ring, with 5IN substantially retaining the efficacy of Nonivamide to protect linoleic and cholesterol against free radical attack. Moreover, the pre-treatment with 5IN, at noncytotoxic concentrations, significantly preserved LDL from Cu(2+)-induced oxidative damage at 37 degrees C for 2h, inhibiting the reduction of polyunsaturated fatty acids and cholesterol and the increase of their oxidative products.
CONCLUSIONS:
The results of the present work suggest that 5IN exerts useful antioxidant properties in different in vitro systems of lipid peroxidation. This, coupled to its lacks of pungency and TRPV1 inhibiting properties, qualifies this phenolic compound as an attractive candidate for further investigations in vivo. | | Eur J Pharm Sci. 2001 Jan;12(3):195-203. | | Capsaicin and nonivamide as novel skin permeation enhancers for indomethacin.[Pubmed: 11113638] |
METHODS AND RESULTS:
The study was conducted in vitro to investigate the changes of indomethacin transdermal permeation pretreated by capsaicin and Nonivamide, two compounds chemically similar to Azone.
The combined effect of low frequency ultrasound (20 kHz) and enhancers on the indomethacin permeation was also evaluated. The experimental data demonstrated that capsaicin and Nonivamide significantly enhanced the flux of indomethacin across nude mouse skin. Enhancement effects of both analogues were very similar and depended predominantly on the concentration tested.
Histological examination coupled with visual scores indicated the safety of capsaicin and Nonivamide on skin structure.
CONCLUSIONS:
Simultaneous application of ultrasound and enhancers significantly increased skin permeation of indomethacin compared with either ultrasound or enhancers alone. Better effect was obtained by the combination with capsaicin than Nonivamide. | | J Cell Biochem . 2015 Jun;116(6):1153-63. | | Nonivamide enhances miRNA let-7d expression and decreases adipogenesis PPARγ expression in 3T3-L1 cells[Pubmed: 25704235] | | Abstract
Red pepper and its major pungent principle, capsaicin (CAP), have been shown to be effective anti-obesity agents by reducing energy intake, enhancing energy metabolism, decreasing serum triacylglycerol content, and inhibiting adipogenesis via activation of the transient receptor potential cation channel subfamily V member 1 (TRPV1). However, the binding of CAP to the TRPV1 receptor is also responsible for its pungent sensation, strongly limiting its dietary intake. Here, the effects of a less pungent structural CAP-analog, Nonivamide, on adipogenesis and underlying mechanisms in 3T3-L1 cells were studied. Nonivamide was found to reduce mean lipid accumulation, a marker of adipogenesis, to a similar extent as CAP, up to 10.4% (P < 0.001). Blockage of the TRPV1 receptor with the specific inhibitor trans-tert-butylcyclohexanol revealed that the anti-adipogenic activity of Nonivamide depends, as with CAP, on TRPV1 receptor activation. In addition, in cells treated with Nonivamide during adipogenesis, protein levels of the pro-adipogenic transcription factor peroxisome-proliferator activated receptor γ (PPARγ) decreased. Results from miRNA microarrays and digital droplet PCR analysis demonstrated an increase in the expression of the miRNA mmu-let-7d-5p, which has been associated with decreased PPARγ levels.
Keywords: 3T3-L1 ADIPOGENESIS; CELL DIFFERENTIATION; LIPID ACCUMULATION; PEROXISOME PROLIFERATOR-ACTIVATED RECEPTOR (PPAR); TRPV1; microRNA; trans-tert-BUTYLCYCLOHEXANOL. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)