| Structure Identification: |
| Chem Pharm Bull (Tokyo). 1997 Apr;45(4):700-5. | | Studies on the constituents of Broussonetia species. II. Six new pyrrolidine alkaloids, broussonetine A, B, E, F and broussonetinine A and B, as inhibitors of glycosidases from Broussonetia kazinoki Sieb.[Pubmed: 9145506 ] |
METHODS AND RESULTS:
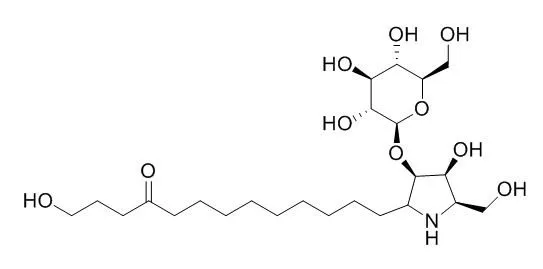
Six new pyrrolidine alkaloids called Broussonetine A, B, E, F, and broussonetinine A and B were isolated from the branches of Broussonetia kazinoki Sieb. (Moraceae). Broussonetine A, B, E and F were formulated as 2 beta-hydroxymethyl-3 beta-hydroxy-5-alpha- (10-oxo-13-hydroxytridecyl)-pyrrolidine-4-O-beta-D-glucopyranoside (1), 2 beta-hydroxymethyl-3 beta-hydroxy-5 alpha-(9-oxo-13-hydroxytridecyl)-pyrrolidine-4-O-beta-D-glucopy ran oside (2), 2 beta-hydroxymethyl-3 alpha,4 beta-dihydroxy-5 alpha-(1,13-dihydroxy-10-oxo-tridecyl)-pyrrolidine (3), and 2 beta-hydroxymethyl-3 alpha,4 beta-dihydroxy-5 alpha-(1,13-dihydroxy-9-oxo-tridecyl)-pyrrolidine (4), respectively.
CONCLUSIONS:
Broussonetinine A and B (5 and 6) were also isolated and identified as the aglycones of 1 and 2. 3 and 4 exhibited a strong inhibition of alpha-glucosidase, beta-glucosidase, beta-galactosidase and beta-mannosidase, while 5 and 6 showed a strong inhibition of beta-galactosidase and alpha-mannosidase. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)