| Structure Identification: |
| Phytochem Anal. 2014 Mar-Apr;25(2):122-6. | | Quantitative analysis of amygdalin and prunasin in Prunus serotina Ehrh. using (1) H-NMR spectroscopy.[Pubmed: 24115144] | Prunus serotina is native to North America but has been invasively introduced in Europe since the seventeenth century. This plant contains cyanogenic glycosides that are believed to be related to its success as an invasive plant. For these compounds, chromatographic- or spectrometric-based (targeting on HCN hydrolysis) methods of analysis have been employed so far. However, the conventional methods require tedious preparation steps and a long measuring time.
To develop a fast and simple method to quantify the cyanogenic glycosides, amygdalin and Prunasin in dried Prunus serotina leaves without any pre-purification steps using (1) H-NMR spectroscopy.
METHODS AND RESULTS:
Extracts of Prunus serotina leaves using CH3 OH-d4 and KH2 PO4 buffer in D2 O (1:1) were quantitatively analysed for amygdalin and Prunasin using (1) H-NMR spectroscopy. Different internal standards were evaluated for accuracy and stability. The purity of quantitated (1) H-NMR signals was evaluated using several two-dimensional NMR experiments.
Trimethylsilylpropionic acid sodium salt-d4 proved most suitable as the internal standard for quantitative (1) H-NMR analysis. Two-dimensional J-resolved NMR was shown to be a useful tool to confirm the structures and to check for possible signal overlapping with the target signals for the quantitation. Twenty-two samples of P. serotina were subsequently quantitatively analysed for the cyanogenic glycosides Prunasin and amygdalin.
CONCLUSIONS:
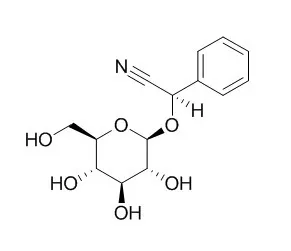
The NMR method offers a fast, high-throughput analysis of cyanogenic glycosides in dried leaves permitting simultaneous quantification and identification of Prunasin and amygdalin in Prunus serotina. | | Plant Physiol. 2012 Apr;158(4):1916-32. | | Prunasin hydrolases during fruit development in sweet and bitter almonds.[Pubmed: 22353576] | Amygdalin is a cyanogenic diglucoside and constitutes the bitter component in bitter almond (Prunus dulcis). Amygdalin concentration increases in the course of fruit formation.
The monoglucoside Prunasin is the precursor of amygdalin. Prunasin may be degraded to hydrogen cyanide, glucose, and benzaldehyde by the action of the β-glucosidase Prunasin hydrolase (PH) and mandelonitirile lyase or be glucosylated to form amygdalin.
METHODS AND RESULTS:
The tissue and cellular localization of PHs was determined during fruit development in two sweet and two bitter almond cultivars using a specific antibody toward PHs. Confocal studies on sections of tegument, nucellus, endosperm, and embryo showed that the localization of the Prunasin hydrolase proteins is dependent on the stage of fruit development, shifting between apoplast and symplast in opposite patterns in sweet and bitter cultivars. Two different Prunasin hydrolase genes, Ph691 and Ph692, have been identified in a sweet and a bitter almond cultivar. Both cDNAs are 86% identical on the nucleotide level, and their encoded proteins are 79% identical to each other. In addition, Ph691 and Ph692 display 92% and 86% nucleotide identity to Ph1 from black cherry (Prunus serotina). Both proteins were predicted to contain an amino-terminal signal peptide, with the size of 26 amino acid residues for PH691 and 22 residues for PH692.
CONCLUSIONS:
The Prunasin hydrolase activity and the localization of the respective proteins in vivo differ between cultivars.
This implies that there might be different concentrations of Prunasin available in the seed for amygdalin synthesis and that these differences may determine whether the mature almond develops into bitter or sweet. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)