| In vitro: |
| Toxicon. 2016 Feb;110:27-34. | | Bufadienolides from parotoid gland secretions of Cuban toad Peltophryne fustiger (Bufonidae): Inhibition of human kidney Na(+)/K(+)-ATPase activity.[Pubmed: 26615828 ] | Parotoid gland secretions of toad species are a vast reservoir of bioactive molecules with a wide range of biological properties.
METHODS AND RESULTS:
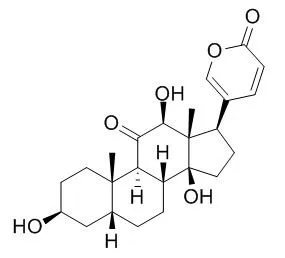
Herein, for the first time, it is described the isolation by preparative reversed-phase HPLC and the structure elucidation by NMR spectroscopy and/or mass spectrometry of nine major bufadienolides from parotoid gland secretions of the Cuban endemic toad Peltophryne fustiger: ψ-Bufarenogin, gamabufotalin, Bufarenogin, arenobufagin, 3-(N-suberoylargininyl) marinobufagin, bufotalinin, telocinobufagin, marinobufagin and bufalin. In addition, the secretion was analyzed by UPLC-MS/MS which also allowed the identification of azelayl arginine. The effect of arenobufagin, bufalin and ψ-Bufarenogin on Na(+)/K(+)-ATPase activity in a human kidney preparation was evaluated. These bufadienolides fully inhibited the Na(+)/K(+)-ATPase in a concentration-dependent manner, although arenobufagin (IC50 = 28.3 nM) and bufalin (IC50 = 28.7 nM) were 100 times more potent than ψ-Bufarenogin (IC50 = 3020 nM).
CONCLUSIONS:
These results provided evidence about the importance of the hydroxylation at position C-14 in the bufadienolide skeleton for the inhibitory activity on the Na(+)/K(+)-ATPase. | | Biocatalysis, 2011, 29(2-3):96-101. | | Biotransformation of arenobufagin and cinobufotalin by Alternaria alternata.[Reference: WebLink] | The biotransformation of arenobufagin (1) and cinobufotalin (2), isolated from the natural medicine Chan Su, by Alternaria alternata AS 3.4578 was carried out.
METHODS AND RESULTS:
Incubation of 1 and 2 afforded six metabolites: 3-oxo-arenobufagin (1a), ψ-Bufarenogin (1b), 3-oxo-ψ-Bufarenogin (1c), 3-oxo-Δ4-derivative of cinobufotalin (2a), 3-oxo-cinobufotalin (2b) and 12β-hydroxycinobufotalin (2c). Among them, metabolites 1a, 1c and 2c are new compounds and their structures were characterized on the basis of their spectroscopic data (NMR, MS and IR).
CONCLUSIONS:
Compounds 1, 2, 1b, 2a and 2b were evaluated for their cytotoxicity against HepG2 and MCF-7 human cancer cells, and all of them showed significant inhibitory activities. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)