| Structure Identification: |
| Modern chinese medicine, 2018. | | Saponin Constituents from Fruits of Panax ginseng.[Reference: WebLink] | To study the saponin constituents of in the fruits of Panax ginseng C. A. Meyer.
METHODS AND RESULTS:
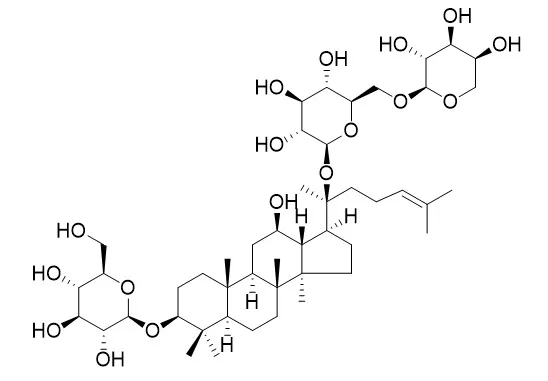
The chemical constituents were isolated and purified by various chromatographic methods including macroporous resin,MCI gel, silica gel columns and semi-preparative HPLC, and their structures were identified by NMR and MS data analysis. Twenty-two compounds were isolated and identified as ginsenoside F2(1),vina-ginsenoside R8(2),pseudoginsenoside Rc1(3),ginsenoside Re(4),notoginsenoside R2(5),ginsenoside F5(6),ginsenoside F3(7),ginsenoside Ia(8),chikusetsusaponin LM1(9),20(S)-ginsenoside Rg2(10),ginsenoside Rc(11),ginsenoside F1(12),ginsenoside Rd(13),ginsenoside Rb2(14),ginsenoside Rb1(15),ginsenoside Rh6(16),ginsenoside Rh4(17),ginsenoside Rh5(18),gypenoside XⅦ(19),gypenoside IX(20),notoginsenoside Fe(21) and Ginsenoside Rd2 (Quinquenoside L10)(22).
CONCLUSIONS:
Compounds 2,3,5,8,9,16,18-22 were isolated from the fruits of P. ginseng for the first time. | | Journal of Asian Natural Products Research, 2009, 11(3):195-201. | | Three new triterpenoid saponins from the leaves and stems of Panax quinquefolium.[Reference: WebLink] | Three new minor dammarane saponins, Ginsenoside Rd2 (Quinquenoside L10) (1), L14 (2), and L16 (3), were isolated from the leaves and stems of Panax quinquefolium L.
METHODS AND RESULTS:
By the combination of one- and two-dimensional NMR techniques and MS spectroscopic analysis, their structures were established as 20-O-(alpha-L-arabinopyranosyl-(1-6)-O-beta-D-glucopyranosyl)-3-O-beta-D-glucopyranosyl-dammar-24-ene-3,12, 20-triol (1), 20-O-alpha-L-arabinopyranosyl-3-O-(beta-D-glucopyranosyl-(1-2)-O-beta-d-glucopyranosyl)-dammar-24-ene-3,12,20-triol (2), 3-O-(beta-D-glucopyranosyl-(1-2)-O-beta-d-glucopyranosyl)-20-O-(beta-D-glucopyranosyl-(1-6)-beta-D-glucopyranosyl)-dammarane-3,12,20,24,25-pentaol (3).
CONCLUSIONS:
The content of artemisinin was significantly enhanced by 3.0 mg l(- 1) of compounds 1, 2, and 3 in the callus of Artemisia annua. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)