| In vitro: |
| Bmc Compl. Altern. M., 2015, 15(1):1-11. | | Calycosin-7-O-β-D-glucoside promotes oxidative stress-induced cytoskeleton reorganization through integrin-linked kinase signaling pathway in vascular endothelial cells.[Pubmed: 26346982 ] | Dysfunction of vascular endothelium is implicated in many pathological situations. Cytoskeleton plays an importance role in vascular endothelial permeability barrier and inflammatory response. Many Chinese herbs have the endothelial protective effect, of which, "Astragalus membranaceus" is a highly valued herb for treatment of cardiovascular and renal diseases in traditional Chinese medicine, In this study, we tested whether calycosin-7-O-β-D-glucoside (Calycosin), a main effective monomer component of "Astragalus membranaceus", could protect endothelial cells from bacterial endotoxin (LPS)-induced cell injury.
METHODS AND RESULTS:
Endothelial cell injury was induced by exposing human umbilical vein endothelial cells (HUVECs) to LPS. The effects of calycosin on LPS-induced changes in cell viability, apoptosis rate, cell migration, nitric oxide synthase (NOS), generationof intracellular reactive oxygen species (ROS) and cytoskeleton organization were determined. Microarray assay was employed to screen the possible gene expression change. Based on the results of microarray assay, the expression profile of genes involved in Rho/ROCK pathway and AKT pathway were further evaluated with quantitative real-time RT-PCR or western blot methods.
Calycosin improved cell viability, suppressed apoptosis and protected the cells from LPS-induced reduction in cell migration and generation of ROS, protein level of NOS at a comparable magnitude to that of Y27632 and valsartan. Similar to Y27632 and valsartan, Calycosin, also neutralized LPS-induced actomyosin contraction and vinculin protein aggregation. Microarray assay, real-time PCR and western blot results revealed that LPS induced expression of FN, ITG A5, RhoA, PI3K (or PIP2 in western blotting), FAK, VEGF and VEGF R2, and inhibited expression of MLCP. We believed multiple pathways involved in the regulation of calycosin on HUVECs. Calycosin are considered to be able to activate MLCP through promoting the generation of NO, decreasing PMLC, suppressing the cytoskeleton remodeling caused by activation of Rho/ROCK pathway and inhibiting AKT pathway by decreasing VEGF, VEGF R2 and PI3K level.
CONCLUSIONS:
Calycosin protected HUVEC from LPS-induced endothelial injury, possibly through suppression of Rho/ROCK pathway and regulation of AKT pathway. | | Neural Plast . 2019 Dec 1;2019:8798069. | | Calycosin-7- O- β- D-glucoside Attenuates OGD/R-Induced Damage by Preventing Oxidative Stress and Neuronal Apoptosis via the SIRT1/FOXO1/PGC-1 α Pathway in HT22 Cells[Pubmed: 31885537] | | Abstract
Neuronal apoptosis induced by oxidative stress is a major pathological process that occurs after cerebral ischemia-reperfusion. Calycosin-7-O-β-D-glucoside (CG) is a representative component of isoflavones in Radix Astragali (RA). Previous studies have shown that CG has potential neuroprotective effects. However, whether CG alleviates neuronal apoptosis through antioxidant stress after ischemia-reperfusion remains unknown. To investigate the positive effects of CG on oxidative stress and apoptosis of neurons, we simulated the ischemia-reperfusion process in vitro using an immortalized hippocampal neuron cell line (HT22) and oxygen-glucose deprivation/reperfusion (OGD/R) model. CG significantly improved cell viability and reduced oxidative stress and neuronal apoptosis. In addition, CG treatment upregulated the expression of SIRT1, FOXO1, PGC-1α, and Bcl-2 and downregulated the expression of Bax. In summary, our findings indicate that CG alleviates OGD/R-induced damage via the SIRT1/FOXO1/PGC-1α signaling pathway. Thus, CG maybe a promising therapeutic candidate for brain injury associated with ischemic stroke. | | Neural Plast . 2019 Dec 1;2019:8798069. | | Calycosin-7- O- β- D-glucoside Attenuates OGD/R-Induced Damage by Preventing Oxidative Stress and Neuronal Apoptosis via the SIRT1/FOXO1/PGC-1 α Pathway in HT22 Cells[Pubmed: 31885537] | | Abstract
Neuronal apoptosis induced by oxidative stress is a major pathological process that occurs after cerebral ischemia-reperfusion. Calycosin-7-O-β-D-glucoside (CG) is a representative component of isoflavones in Radix Astragali (RA). Previous studies have shown that CG has potential neuroprotective effects. However, whether CG alleviates neuronal apoptosis through antioxidant stress after ischemia-reperfusion remains unknown. To investigate the positive effects of CG on oxidative stress and apoptosis of neurons, we simulated the ischemia-reperfusion process in vitro using an immortalized hippocampal neuron cell line (HT22) and oxygen-glucose deprivation/reperfusion (OGD/R) model. CG significantly improved cell viability and reduced oxidative stress and neuronal apoptosis. In addition, CG treatment upregulated the expression of SIRT1, FOXO1, PGC-1α, and Bcl-2 and downregulated the expression of Bax. In summary, our findings indicate that CG alleviates OGD/R-induced damage via the SIRT1/FOXO1/PGC-1α signaling pathway. Thus, CG maybe a promising therapeutic candidate for brain injury associated with ischemic stroke. |
|
| In vivo: |
| Mol Med Rep. 2016 Jan; 13(1): 633–640. | | Calycosin-7-O-β-d-glucoside attenuates ischemia-reperfusion injury in vivo via activation of the PI3K/Akt pathway[Pubmed: 26648122] | The aim of the present study was to investigate the effects and mechanisms of calycosin‑7‑O‑β‑D‑glucoside (CG) on ischemia‑reperfusion (I/R) injury in vivo.
METHODS AND RESULTS:
Hemodynamic parameters, including ejection fraction (EF), fractional shortening (FS), left ventricular end‑systolic pressure (LVESP) and left ventricular end‑diastolic pressure (LVEDP) were monitored using an ultrasound system, and infarct size was measured using Evans blue/tetrazolium chloride double staining. The activities of serum creatine kinase (CK), lactate dehydrogenase (LDH) and superoxide dismutase (SOD), and the levels of malondialdehyde (MDA) were determined to assess the degree of myocardial injury and oxidative stress‑induced damage. The protein expression levels of cleaved‑caspase‑3, cleaved‑caspase‑9, phosphorylated (p)‑phosphatidylinositol 3‑kinase (PI3K) p85, PI3K p85, p‑Akt and Akt were determined using western blotting. The results demonstrated that pretreatment with high dose (H)‑CG markedly improved cardiac function, as evidenced by upregulated EF, FS and LVESP, and downregulated LVEDP. In addition, administration of CG resulted in significant decreases in infarct size in the I/R+low dose‑CG and I/R+H‑CG groups, compared with the I/R group. The activities of CK and LDH, and the levels of MDA in the I/R+H‑CG group were reduced, compared with those in the I/R group, whereas SOD activity was elevated. Treatment with CG inhibited the cleavage and activity of caspase‑3 and caspase‑9, and enhanced the phosphorylation of PI3K p85 and Akt. Notably, administration of the PI3K inhibitor, LY294002, markedly lowered the levels of p‑PI3K p85/p‑Akt, and eradicated the inhibitory effects of H‑CG on infarct size, myocardial injury and oxidative stress‑induced damage.
CONCLUSIONS:
Taken together, the results suggested that CG may alleviate I/R injury by activating the PI3K/Akt signaling pathway. |
|


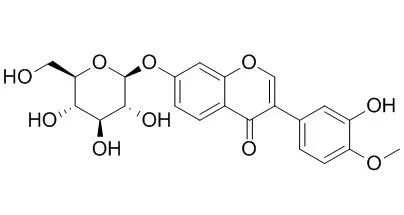



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)