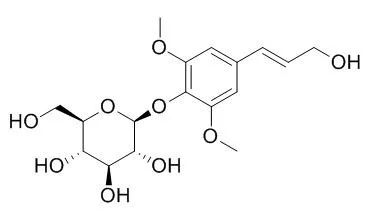
| Description: |
Syringin (Eleutheroside B) has neuroprotective, tonic, adaptogenic, antitumour, anti- platelet aggregation, anti-inflammatory, antinociceptive ,and immune-modulating properties. It reduced the expression levels of inducible NO synthase (iNOS) ,COX,TNF-α, Beta Amyloid, and Caspase. |
| Targets: |
TNF-α | Beta Amyloid | Caspase | COX | NOS |
| In vitro: |
| Arch Pharm Res. 2010 Apr;33(4):531-8. | | Syringin from stem bark of Fraxinus rhynchophylla protects Abeta(25-35)-induced toxicity in neuronal cells.[Pubmed: 20422361] | The medicinal herb Jinpi, derived from the dried stem barks of Fraxinus rhynchophylla belonging to Oleaceae is widely used as a variety of Korean folk remedies for anti-inflammatory, febricide, antidiarrhea, and antileukorrhea diseases.
METHODS AND RESULTS:
In the course of screening antidementia agents from natural products, F. rhynchophylla showed significant inhibitory activity toward Abeta(25-35)-induced neuronal cell death. An active principle was isolated and identified as Syringin.When the neuroblastoma cells were exposed to 50 microM Abeta(25-35), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction rate (survival rate) decreased to 60.21 +/- 2.16% over control while Syringin treated ones recovered cell viability up to 79.12 +/- 1.39% at 20 microM. In addition, 20 microM Syringin almost completely removed Abeta(25-35)-induced reactive oxygen species. The neuroprotective effect of Syringin seemed to be originated from the reduction of apoptosis since decrease in caspase-3 activity and expression, reduction in cleaved PARP, and DNA fragmentation were observed.
CONCLUSIONS:
These results suggest that F. rhynchophylla and Syringin are expected to be useful for preventing Abeta(25-35)-induced neuronal cell damage. |
|
| In vivo: |
| Fundam Clin Pharmacol. 2015 Apr;29(2):178-84. | | Syringin may exert sleep-potentiating effects through the NOS/NO pathway.[Pubmed: 25377727] | Sleep is essential for basic survival as well as for optimal physical and cognitive performance in both human beings and animals.
METHODS AND RESULTS:
To investigate the effect of Syringin on sleep of anesthetized mice and the potential mechanisms, 35 male Kunming mice were randomly divided into six experimental groups (n = 5) and one control group (n = 5). Sleep latency and sleep duration, as well as nitric oxide (NO) content and nitric oxide synthase (NOS) activity, were determined after Syringin administration. The NO precursor l-Arginine (l-Arg) or NOS inhibitor NG-Nitro-l-arginine methyl ester (l-NAME) was administered alone or in combination with Syringin, and time for sleep latency and duration was recorded. After intragastric administration of Syringin, sleep latency decreased in a dose- and time-dependent manner, concomitant with increased sleep duration. The optimal sleep performance was obtained when Syringin was given at a dose of 80 mg/kg for eight consecutive days. Syringin significantly reduced NO concentration and NOS activity. Administration of l-Arg prolonged sleep latency and shortened sleep duration, and the effects were fully reversed by Syringin coadministration. Administration of L-NAME induced a significant reduction in sleep latency and a corresponding increase in sleep duration, and coadministration of Syringin further enhanced the effects.
CONCLUSIONS:
The finding of our study demonstrated that Syringin could exert sleep-potentiating effects on anesthetized mice in a time- and dose-dependent manner, and these effects may be intimately correlated with the NO/NOS pathway. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)