| In vitro: |
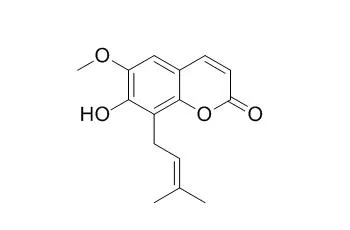
| Medicinal Chemistry Research, 2016, 25(3):466-472. | | Cytotoxic constituents of Oldenlandia umbellata and isolation of a new symmetrical coumarin dimer.[Reference: WebLink] | Chemical investigation of HUM-E and HUM-B resulted in the isolation of a novel symmetrical coumarin dimer named oledicoumarin (1), together with eleven known compounds, hedyotiscone B (2), Cedrelopsin (3), pheophorbide A methyl ester (4), deacetyl asperuloside (5), scandoside methyl ester (6), asperulosidic acid (7), scandoside (8), nicotinic acid (9), 6α-hydroxy geniposide (10) anthragallol 1,2-dimethyl ether (11) and anthragallol 1,3-dimethyl ether (12).
METHODS AND RESULTS:
All compounds were isolated for the first time from O. umbellata except anthragallols. This is the foremost report exploring the presence of coumarin derivatives in O. umbellata. Testing of cytotoxicity of isolated constituents revealed that compounds 3, 4, 11 and 12 showed significant inhibition against A549 cells with IC50 values of 3.6–5.9 μg/mL. Compounds 4, 11 and 12 showed marked inhibitory effect against MDA-MB-231 cells (IC50 3.6–9.1 μg/mL). Compounds 4 (IC50 1.7 μg/mL) and 7 (IC50 6.1 μg/mL) were highly active against HT-29 cells.
CONCLUSIONS:
In summary, the less polar fraction of O. umbellata and its constituents were found to be cytotoxic. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)