| Structure Identification: |
| Bioorg Med Chem Lett. 2015 Aug 6. pii: S0960-894X(15)00825-2. | | A novel sesquiterpene and three new phenolic compounds from the rhizomes of Acorus tatarinowii Schott.[Pubmed: 26296476] |
METHODS AND RESULTS:
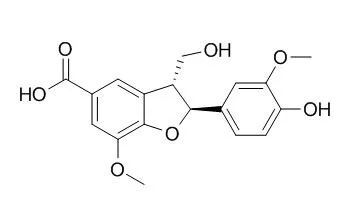
A novel sesquiterpene with an unprecedented epoxy lactone skeleton, named tatarinolactone, together with two new amides, a new biphenylpropanoid and two known lignans were isolated from the rhizomes of Acorus tatarinowii Schott. Their structures were identified as 6,7,8-trihydroxy-4α-isobutyl-4,7-dimethylhexahydro-6,8α-epoxychromen-2(3H)-one (1), (E)-methyl 4-[3-(4-hydroxy-3-methoxyphenyl)acrylamido]butanoate (2), (Z)-methyl 4-[3-(4-hydroxy-3-methoxyphenyl)acrylamido]butanoate enol isomer (3), (R)-4-hydroxy-3-[1-hydroxy-3-(4-hydroxy-3-methoxyphenyl)propan-2-yl]-5-methoxybenzoic acid (4), (2S,3R)-Ceplignan (5), and (2R,3S)-Ceplignan (6), respectively, based on extensive spectroscopic analysis and by comparison to the known compounds. To test their effects on serotonin transporters, a high content assay using hSERT-HEK293 cell line was adopted.
CONCLUSIONS:
Results indicated that compounds 1 and 4 significantly inhibited SERT activity, while compounds 2, 3, 5, and 6 significantly enhanced SERT activity, which partly explain the traditional uses of the rhizomes of Acorus tatarinowii Schott in treatments of neuropsychiatric and digestive disorders. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)