| In vitro: |
| Beilstein J Org Chem. 2007 Jan 31;3:3. | | Cimicifoetisides A and B, two cytotoxic cycloartane triterpenoid glycosides from the rhizomes of Cimicifuga foetida, inhibit proliferation of cancer cells.[Pubmed: 17266751 ] |
METHODS AND RESULTS:
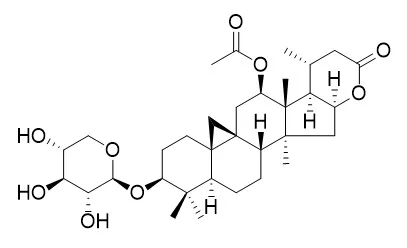
Two new cycloartane-type triterpene glycosides, namely cimicifoetisides A (1) and B (2), along with seven known compounds cimigenol, 25-O-acetylcimigenol, cimigenol 3-O-beta-D-xylopyranoside, 12beta-hydroxycimigenol 3-O-beta-D-xylopyranoside, cimigenol 3-O-alpha-L-arabinopyranoside, 25-deoxyshengmanol 3-O-beta-D-xylopyranoside and Cimilactone A, were isolated from the rhizomes of Cimicifuga foetida. Their structures were elucidated as cimigenol 3-O-(2'-O-acetyl)-alpha-L-arabinopyranoside (1) and 25-O-acetylcimigenol 3-O-(2'-O-acetyl)-alpha-L-arabinopyranoside (2).
CONCLUSIONS:
Both compounds 1 and 2 exhibited potent cytotoxicity against rat EAC (Ehrlich ascites carcinoma) and MDA-MB-A231 (human breast cancer) cells with IC50 values of 0.52 and 6.74 microM for 1, and 0.19 and 10.21 microM for 2, suggesting their potential for further investigation as anti-cancer agents. | | Phytother Res. 2006 Nov;20(11):945-8. | | Anticomplement activity of cycloartane glycosides from the rhizome of Cimicifuga foetida.[Pubmed: 16906637] |
METHODS AND RESULTS:
A tetranor-cycloartane glycoside and two 9,19-cycloartane glycosides were isolated from the EtOAc-soluble fraction of the rhizome of Cimicifuga foetida. The structures of the compounds were determined to be Cimilactone A (1), 25-O-acetylcimigenol 3-O-beta-d-xylopyranoside (2) and cimigenol 3-O-alpha-l-arabinopyranoside (3), respectively, using spectroscopic analysis. The three compounds were examined for their anticomplement activity against the classical pathway of the complement system.
CONCLUSIONS:
Compound 1 showed significant anticomplement activity with an IC(50) value of 28.6 microm, whereas compounds 2 and 3 were inactive. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)