| Description: |
Vitexicarpin has shown antitumor, cytotoxicity, anti-inflammatory, analgesic and immunoregulatory properties.Vitexicarpin can act as a novel angiogenesis inhibitor, it exerts good antiangiogenic effects by inhibiting vascular-endothelial-growth-factor-(VEGF-) induced endothelial cell proliferation, migration, and capillary-like tube formation on matrigel in a dose-dependent manner. It can significantly reduce vascular inflammation, through inhibition of ROS-NF-κB pathway in vascular endothelial cells. |
| In vitro: |
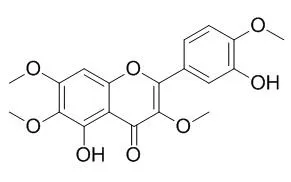
| Asian Pac J Cancer Prev. 2012;13(12):6369-74. | | Vitexicarpin induces apoptosis in human prostate carcinoma PC-3 cells through G2/M phase arrest.[Pubmed: 23464460] | Vitexicarpin (3', 5-dihydroxy-3, 4', 6, 7-tetramethoxyflavone), a polymethoxyflavone isolated from Viticis Fructus (Vitex rotundifolia Linne fil.), has long been used as an anti-inflammatory herb in traditional Chinese medicine. It has also been reported that Vitexicarpin can inhibit the growth of various cancer cells. However, there is no report elucidating its effect on human prostate carcinoma cells.
METHODS AND RESULTS:
The aim of the present study was to examine the apoptotic induction activity of Vitexicarpin on PC-3 cells and molecular mechanisms involved. MTT studies showed that Vitexicarpin dose-dependently inhibited growth of PC-3 cells with an IC50~28.8 μM. Hoechst 33258 staining further revealed that Vitexicarpin induced apoptotic cell death. The effect of Vitexicarpin on PC-3 cells apoptosis was tested using prodium iodide (PI)/Annexin V-FITC double staining and flow cytometry. The results indicated that Vitexicarpin induction of apoptotic cell death in PC-3 cells was accompanied by cell cycle arrest in the G2/M phase. Furthermore, our study demonstrated that Vitexicarpin induction of PC-3 cell apoptosis was associated with upregulation of the proapoptotic protein Bax, and downregulation of antiapoptotic protein Bcl-2, release of Cytochrome c from mitochondria and decrease in mitochondrial membrane potential.
CONCLUSIONS:
Our findings suggested that Vitexicarpin may become a potential leading drug in the therapy of prostate carcinoma. | | Planta Med. 2002 Nov;68(11):1047-9. | | Tracheospasmolytic activity of viteosin-A and vitexicarpin isolated from vitex trifolia.[Pubmed: 12451502] |
METHODS AND RESULTS:
The n-hexane extract that has shown activity in the tracheospasmolytic bioassay was fractionated by solvent extraction and from the major active fraction two compounds were isolated and identified as viteosin-A and Vitexicarpin. These compounds blocked spontaneous contraction of isolated male guinea pig trachea induced by histamine; however only Vitexicarpin was active in a model using sensitized guinea pig trachea stimulated by ovalbumin up to minimum dose of 1.3 x 10(-5) M.
CONCLUSIONS:
The result suggests that Vitexicarpin is able to block effects of histamine released from sensitized mast cells possibly by stabilizing the mast cells membrane function. | | Oncotarget . 2017 Apr 27;8(34):56267-56280. | | Casticin attenuates liver fibrosis and hepatic stellate cell activation by blocking TGF-β/Smad signaling pathway[Pubmed: 28915589] | | Abstract
Although many advances have been made in understanding the pathogenesis of liver fibrosis, few options are available for treatment. Casticin, one of the major flavonoids in Fructus Viticis extracts, has shown hepatoprotective potential, but its effects on liver fibrosis are not clear. In this study, we investigated the antifibrotic activity of casticin and its underlying mechanism in vivo and in vitro. Male mice were injected intraperitoneally with carbon tetrachloride (CCl4) or underwent bile duct ligation (BDL) to induce liver fibrosis, followed by treatment with casticin or vehicle. In addition, transforming growth factor-β1(TGF-β1)-activated LX-2 cells were used. In vivo experiments showed that treatment with casticin alone had no toxic effect while significantly attenuating CCl4-or BDL-induced liver fibrosis, as indicated by reductions in the density of fibrosis, hydroxyproline content, expression of α-SMA and collagen α1(I) mRNA. Moreover, casticin inhibited LX2 proliferation, induced apoptosis in a time- and dose-dependent manner in vitro. The underlying molecular mechanisms for the effect of casticin involved inhibition of hepatic stellate cell (HSC) activation and reduced the expression of matrix metalloproteinase (MMP)-2, MMP-9, tissue inhibitor of metalloproteinases (TIMP)-1 and TIMP-2 resulting from blocking TGF-β1/Smad signaling, as well as increased the apoptosis of HSCs. The results suggest that casticin has potential benefits in the attenuation and treatment of liver fibrosis.
Keywords: CCl4; TGF-β/Smad; casticin; hepatic stellate cell; liver fibrosis. | | J Cell Biochem . 2019 Jun;120(6):9787-9798. | | Casticin inhibits growth and enhances ionizing radiation-induced apoptosis through the suppression of STAT3 signaling cascade[Pubmed: 30520154] | | Abstract
Casticin (CTC), one of the major components of Vitex rotundifolia L., has been reported to exert significant beneficial pharmacological activities and can function as an antiprolactin, anticancer, anti-inflammatory, neuroprotective, analgesic, and immunomodulatory agent. This study aimed at investigating whether the proapoptotic effects of CTC may be mediated through the abrogation of signal transducers and activators of transcription-3 (STAT3) signaling pathway in a variety of human tumor cells. We found that CTC significantly decreased cell viability in a concentration-dependent manner and suppressed cell proliferation in 786-O, YD-8, and HN-9 cells. CTC also induced programmed cell death that was found to be mediated via caspase-3 activation and induction of poly(ADP-ribose) polymerase cleavage. Interestingly, CTC repressed both constitutive and interleukin-6-induced STAT3 activation in 786-O and YD-8 cells but only affected constitutive STAT3 phosphorylation in HN-9 cells. Moreover, CTC could potentiate ionizing radiation-induced apoptotic effects leading to the downregulation of STAT3 activation and thus may be used in combination with radiation against diverse malignancies.
Keywords: apoptosis; cancer; casticin; radiation; signal transducers and activators of transcription-3. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)