| In vitro: |
| Chemical & Pharmaceutical Bulletin, 2008, 33(8):3142-3152. | | Tannins and related compounds. XXXI. Isolation and characterization of proanthocyanidins in Kandelia candel (L.) Druce.[Reference: WebLink] |
METHODS AND RESULTS:
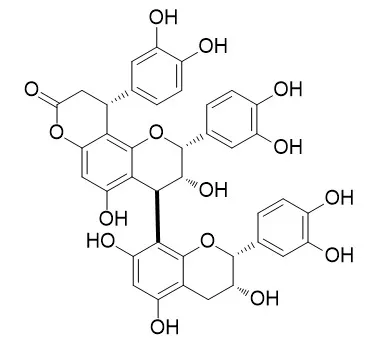
One of the species of commercially available catuaba was identified as Anemopaegma arvense by comparison of its micromorphological characteristics and TLC profile with six species of authenticated plants that are commonly referred to as catuaba. The bioactivity-guided fractionation of the ethyl acetate extract of the stem bark of this catuaba sample resulted in the isolation of one new (1, catuabin A) and three known flavan-3-ol type phenylpropanoids, cinchonain Ia (2), Cinchonain IIa (3), and kandelin A1 (4) with antioxidant activities.
CONCLUSIONS:
The structures of these compounds were determined by a combination of spectroscopic techniques. Additionally, these compounds were tested for their anti-inflammatory, cytotoxicity, antimalarial, and antimicrobial activities, where no activity was observed. | | Journal of the Brazilian Chemical Society, 2011, 22(11):2087-2093. | | Phenylpropanoid Substituted Flavan-3-ols from Trichilia catigua and their In Vitro Antioxidative Activity[Reference: WebLink] |
METHODS AND RESULTS:
The new phenylpropanoid substituted flavan-3-ol apocynin E, together with eight known compounds, epicatechin, procyanidin B2, procyanidin B4, procyanidin C1, cinchonain Ia, cinchonain Ib, cinchonain IIb, and Cinchonain IIa were isolated from an acetone-H2O extract of the air-dried stem bark of Trichilia catigua. The cinchonain Ia e Ib were reevaluated to its estereochemistry. All the compounds were characterized by spectroscopic analysis including 1D and 2D nuclear magnetic resonance (NMR) and mass spectrometry (MS) of their peracetate derivatives. The absolute configuration of the phenylpropanoid moiety was determined by circular dichroism (CD) spectra and by analyzing the anisotropic effects in the Dreiding model and nuclear Overhauser effect (NOESY NMR) experiments.
CONCLUSIONS:
The nine isolated compounds showed higher radical scavenging activity and reducing power than ascorbic acid and Trolox in the free-radical 2,2-diphenyl-1-picrylhydrazyl and Fe3+-Fe2+ reduction assay systems. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)