| In vitro: |
| Food Chem. 2016 Oct 1;208:61-7 | | Antioxidants and α-glucosidase inhibitors from Ipomoea batatas leaves identified by bioassay-guided approach and structure-activity relationships.[Pubmed: 27132824] |
Sweet potato (Ipomoea batatas) leaf (SPL) is an underused commercial vegetable with considerable bio-activities.
METHODS AND RESULTS:
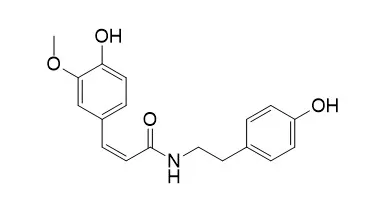
By means of DPPH scavenging ability and α-glucosidase inhibitory oriented isolation, 9 and 7 compounds were isolated and identified, respectively. Among them, trans-N-(p-coumaroyl)tyramine (1), trans-N-feruloyltyramine (2), Cis-N-Feruloyltyramine (3), 4,5-feruloylcourmaoylquinic acid (8), caffeic acid ethyl ester (10), 7-hydroxy-5-methoxycoumarin (11), 7,3'-dimethylquercetin (13) and indole-3-carboxaldehyde (15), were firstly identified from SPL, and four of them (1, 2, 3 and 10) were firstly identified from genus Ipomoea. Phenethyl cinnamides and 3,4,5-triCQA exhibited the strongest α-glucosidase inhibition, while 3,4,5-triCQA and diCQAs were the dominant antioxidants.
CONCLUSIONS:
Structure-activity relationship revealed that higher caffeoylation of quinic acid and lower methoxylation of flavonols resulted in stronger antioxidant activity, and methylation and cis-configuration structure of phenethyl cinnamides weaken the α-glucosidase inhibition. Aforementioned results could help to explain the antioxidant activity and anti-diabetic activity of SPL, and provide theoretical basis for its further application. | | Planta Med. 2003 Jul;69(7):667-72. | | A cytotoxic butenolide, two new dolabellane diterpenoids, a chroman and a benzoquinol derivative formosan Casearia membranacea.[Pubmed: 12898426] |
METHODS AND RESULTS:
Investigation of a cytotoxic chloroform-soluble fraction of the stem of Casearia membranacea (Flacourtiaceae) led to the isolation of five new compounds, including one butenolide, casealactone (1), one chroman, caseamemin (2), two dolabellane diterpenoids, casearimene A (3) and casearimene B (4), one benzoquinol ether, casearinone (5), together with fifteen known compounds, including two amides, N- trans-feruloyltyramine (6) and Cis-N-Feruloyltyramine (7), six steroids, beta-sitosterol (8), stigmast-5-ene-3beta,7alpha-diol (9), stigmast-5-ene-3beta,7beta-diol (10), stigmastane-3beta,5alpha,6beta-triol (11), beta-sitostenone (12), beta-sitosterol 3- O-beta-glucoside (13), two triterpenoids, squalene (14) and friedelin (15), one lignan, (+/-)-syringaresinol (16), two benzenoids, syringaldehyde (17) and vanillic acid (18), one ester, methyl hexadecanoate (19), and anthraquinone (20), respectively.
CONCLUSIONS:
Among these isolates, 1 showed cytotoxicity against P-388 and HT-29 cancer cell lines in vitro, and 6 and 7 showed cytotoxicity against the P-388 cancer cell line. The structures of these compounds were determined by means of spectroscopic techniques, and the structure of 3 was confirmed by X-ray crystallographic analysis. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)