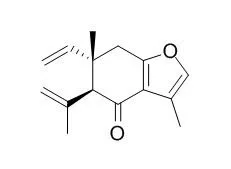
Bioassay-guided isolation of the active hexane fractions of Curcuma zedoaria led to the identification of five pure compounds, namely, Curzerenone (1), neocurdione (2), curdione (3), alismol (4), and zederone (5) and a mixture of sterols, namely, campesterol (6), stigmasterol (7), and β -sitosterol (8). Alismol has never been reported to be present in Curcuma zedoaria.
METHODS AND RESULTS:
All isolated compounds except (3) were evaluated for their cytotoxic activity against MCF-7, Ca Ski, and HCT-116 cancer cell lines and noncancer human fibroblast cell line (MRC-5) using neutral red cytotoxicity assay. Curzerenone and alismol significantly inhibited cell proliferation in human cancer cell lines MCF-7, Ca Ski, and HCT-116 in a dose-dependent manner. Cytological observations by an inverted phase contrast microscope and Hoechst 33342/PI dual-staining assay showed typical apoptotic morphology of cancer cells upon treatment with Curzerenone and alismol. Both compounds induce apoptosis through the activation of caspase-3. It can thus be suggested that Curzerenone and alismol are modulated by apoptosis via caspase-3 signalling pathway. The findings of the present study support the use of Curcuma zedoaria rhizomes in traditional medicine for the treatment of cancer-related diseases.
CONCLUSIONS:
Thus, two naturally occurring sesquiterpenoids, Curzerenone and alismol, hold great promise for use in chemopreventive and chemotherapeutic strategies. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)