| In vitro: |
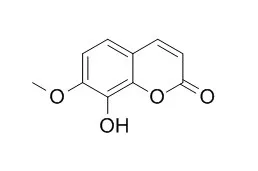
| Article first published online: 17 JUN 2015 | | In Vitro Evaluation of the Effect of 7-Methyl Substitution on Glucuronidation of Daphnetin: Metabolic Stability, Isoform Selectivity, and Bioactivity Analysis[Reference: WebLink] | The C-8 phenol group is essential to exert the bioactivities of daphnetin, but it is readily conjugated with glucuronic acid prior to excretion.
METHODS AND RESULTS:
In this study, Daphnetin 7-methyl ether (7M-DNP) was used to investigate the effect of 7-methyl substitution on daphnetin glucuronidation in human/rat liver (HLM/RLM) and intestine (HIM/RIM) microsomes, and recombinant UDP-glucuronosyltransferases (UGTs). Compared with daphnetin, the Vmax/Km values of 7M-DNP via 8-O-glucuronidation were 2.1-fold lower in HLM, 1.7-fold lower in HIM, and 2.4-fold lower in RLM, suggesting an improvement in metabolic stability. Different from daphnetin 8-O-glucuronidation exclusively catalyzed by UGT1A6 and UGT1A9, UGT1A1, -1A3, -1A7, -1A8, and -1A9 showed glucuronidation activity toward Daphnetin 7-methyl ether . Kinetics studies, chemical inhibition, and the relative activity factor approach were used to demonstrate that UGT1A9 was mainly responsible for the reaction in HLM, whereas UGT1A1 was a primary contributor in HIM. The Vmax/Km values of Daphnetin 7-methyl ether glucuronidation in HLM and HIM were 0.61–0.74-fold lower than those of rat, suggesting the differences between the two species.
CONCLUSIONS:
The bioactivity analysis demonstrated that Daphnetin 7-methyl ether had an anti-inflammatory activity comparable to that of daphnetin. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)