| In vitro: |
| J Ethnopharmacol. 2013 Mar 7;146(1):417-22. | | Zanthoxylum capense constituents with antimycobacterial activity against Mycobacterium tuberculosis in vitro and ex vivo within human macrophages.[Pubmed: 23337743] |
METHODS AND RESULTS:
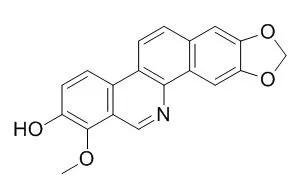
The best results were obtained for a benzophenanthridine alkaloid, Decarine (1), and an N-isobutylamide, N-isobutyl-(2E,4E)-2,4-tetradecadienamide (15), which showed high activity against Mycobacterium tuberculosis H37Rv (MIC of 1.6 μg/ml), and a low macrophage cytotoxicity (IC50>60 μg/ml), indicating considerable selective activity. The benzophenanthridine alkaloid 6-acetonyldihydronitidine (6) revealed cytotoxicity (IC50 1.7 μg/ml), despite the determined MIC of 6.2-12.5 μg/ml. In infected macrophages, Decarine (1) was able to reduce bacterial survival by almost two log units at a concentration of 6.2 μg/ml 5 days post-drug exposure. Compound 15 exhibited an intermediate activity at drug concentrations ranging from 6.2 to 25 μg/ml.
CONCLUSIONS:
The high antimycobacterial activity of Decarine found, both in vitro and ex vivo against mycobacteria, and the low cytotoxicity towards human macrophages indicate that it may be valuable as a lead scaffold for the development of anti-TB drugs. | | Int J Mol Sci. 2013 Nov 13;14(11):22395-408. | | New benzo[c]phenanthridine and benzenoid derivatives, and other constituents from Zanthoxylum ailanthoides: Effects on neutrophil pro-inflammatory responses.[Pubmed: 24232457] |
METHODS AND RESULTS:
A new benzo[c]phenanthridine, oxynorchelerythrine (1), and two new benzenoid derivatives, methyl 4-(2-hydroxy-4-methoxy-3-methyl-4-oxobutoxy)benzoate (2) and (E)-methyl 4-(4-((Z)-3-methoxy-3-oxoprop-1-enyl)phenoxy)-2-methylbut-2-enoate (3), have been isolated from the twigs of Zanthoxylum ailanthoides, together with 11 known compounds (4-14). The structures of these new compounds were determined through spectroscopic and MS analyses.
CONCLUSIONS:
Among the isolated compounds, Decarine (4), (-)-syringaresinol (6), (+)-episesamin (8), glaberide I (9), (-)-dihydrocubebin (10), and xanthyletin (11) exhibited potent inhibition (IC50 values ≤ 4.79 µg/mL) of superoxide anion generation by human nutrophils in response to N-formyl-L-methionyl-L-leucyl-L-phenylalanine/cytochalasin B (fMLP/CB). Compounds 4, 8, and 11 also inhibited fMLP/CB-induced elastase release with IC50 values ≤ 5.48 µg/mL. | | Planta Med. 2012 Jan;78(2):148-53. | | Antibacterial benzofuran neolignans and benzophenanthridine alkaloids from the roots of Zanthoxylum capense.[Pubmed: 22002848] |
METHODS AND RESULTS:
Two new 2-arylbenzofuran neolignans and a new benzophenanthridine alkaloid, together with six known benzophenanthridine alkaloids, namely, Decarine, norchelerythrine, dihydrochelerythrine, 6-acetonyldihydrochelerythrine, tridecanonchelerythrine, and 6-acetonyldihydronitidine, have been isolated from the MeOH extract of the roots of Zanthoxylum capense. Their structures were elucidated by means of spectroscopic techniques including 2D NMR experiments. All the isolated compounds were evaluated for their in vitro antibacterial activity against gram-positive and gram-negative bacteria.
CONCLUSIONS:
Some compounds showed significant inhibitory activity against Staphylococcus aureus ATCC 6538 with MIC values ranging from 12.5 to 50 μg/mL.
|
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)