| Description: |
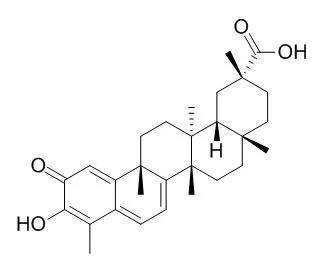
Celastrol is a potent proteasome inhibitor for the chymotrypsin-like activity of a purified 20S proteasome with IC50 of 2.5 μM. Celastrol is also a novel HSP90 inhibitor, it has anti-proliferative, anti-inflammatory, anti-tumor , antiangiogenesis, and antioxidant activities. Celastrol inhibits Plasmodium falciparum enoyl-acyl carrier protein reductase and inhibits VEGF receptors expression. |
| In vitro: |
| Am J Chin Med. 2015;43(1):137-48. | | Celastrol induces mitochondria-mediated apoptosis in hepatocellular carcinoma bel-7402 cells.[Pubmed: 25657108] | Celastrol is a natural terpenoid isolated from Tripterygium wilfordii, a well-known Chinese medicinal herb that presents anti-proliferative activities in several cancer cell lines.
METHODS AND RESULTS:
Here, we investigated whether Celastrol induces apoptosis on hepatocellular carcinoma Bel-7402 cells and further explored the underlying molecular mechanisms. Celastrol caused a dose- and time-dependent growth inhibition and apoptosis of Bel-7402 cells. It increased apoptosis through the up-regulation of Bax and the down-regulation of Bcl-2 in Bel-7402 cells. Moreover, Celastrol induced the release of cytochrome c and increased the activation of caspase-3 and caspase-9, suggesting that Celastrol-induced apoptosis was related to the mitochondrial pathway.
CONCLUSIONS:
These results indicated that Celastrol could induce apoptosis in Bel-7402 cells, which may be associated with the activation of the mitochondria-mediated pathway. | | PLoS One. 2014 Nov 10;9(11):e112470. | | Celastrol stimulates hypoxia-inducible factor-1 activity in tumor cells by initiating the ROS/Akt/p70S6K signaling pathway and enhancing hypoxia-inducible factor-1α protein synthesis.[Pubmed: 25383959] | Celastrol, a tripterine derived from the traditional Chinese medicine plant Tripterygium wilfordii Hook F. ("Thunder of God Vine"), has been reported to have multiple effects, such as anti-inflammation, suppression of tumor angiogenesis, inhibition of tumor growth, induction of apoptosis and protection of cells against human neurodegenerative diseases. However, the mechanisms that underlie these functions are not well defined.
METHODS AND RESULTS:
In this study, we reported for the first time that Celastrol could induce HIF-1α protein accumulation in multiple cancer cell lines in an oxygen-independent manner and that the enhanced HIF-1α protein entered the nucleus and promoted the transcription of the HIF-1 target genes VEGF and Glut-1. Celastrol did not influence HIF-1α transcription. Instead, Celastrol induced the accumulation of the HIF-1α protein by inducing ROS and activating Akt/p70S6K signaling to promote HIF-1α translation. In addition, we found that the activation of Akt by Celastrol was transient. With increased exposure time, inhibition of Hsp90 chaperone function by Celastrol led to the subsequent depletion of the Akt protein and thus to the suppression of Akt activity. Moreover, in HepG2 cells, the accumulation of HIF-1α increased the expression of BNIP3, which induced autophagy. However, HIF-1α and BNIP3 did not influence the cytotoxicity of Celastrol because the main mechanism by which Celastrol kills cancer cells is through stimulating ROS-mediated JNK activation and inducing apoptosis. Furthermore, our data showed that the dose required for Celastrol to induce HIF-1α protein accumulation and enhance HIF-1α transcriptional activation was below its cytotoxic threshold.
CONCLUSIONS:
A cytotoxic dose of Celastrol for cancer cells did not display cytotoxicity in LO2 normal human liver cells, which indicated that the novel functions of Celastrol in regulating HIF-1 signaling and inducing autophagy might be used in new applications, such as in anti-inflammation and protection of cells against human neurodegenerative diseases. | | Bioorg Med Chem. 2014 Nov 1;22(21):6053-61. | | Celastrol inhibits Plasmodium falciparum enoyl-acyl carrier protein reductase.[Pubmed: 25284249] | Enoyl-acyl carrier protein reductase (ENR), a critical enzyme in type II fatty acid biosynthesis, is a promising target for drug discovery against hepatocyte-stage Plasmodium falciparum.
METHODS AND RESULTS:
In order to identify PfENR-specific inhibitors, we docked 70 FDA-approved, bioactive, and/or natural product small molecules known to inhibit the growth of whole-cell blood-stage P. falciparum into several PfENR crystallographic structures. Subsequent in vitro activity assays identified a noncompetitive low-micromolar PfENR inhibitor, Celastrol, from this set of compounds. |
|
| In vivo: |
| Int J Mol Med . 2016 May;37(5):1229-38. | | Celastrol attenuates oxidative stress in the skeletal muscle of diabetic rats by regulating the AMPK-PGC1α-SIRT3 signaling pathway[Pubmed: 27049825] | | Abstract
Oxidative stress plays a key role in the pathogenesis of diabetic myopathy. Celastrol provides a wide range of health benefits, including antioxidant, anti-inflammatory and antitumor effects. We hypothesized that Celastrol may exert an antioxidant effect in the skeletal muscle of diabetic rats. In the present study, MnSOD activity was determined by spectrophotometry. The protein levels were evaluated by western blot analysis and mRNA content was quantified by RT‑qPCR. We firstly found that the levels of AMP-activated protein kinase (AMPK), peroxisome proliferator-activated receptor coactivator 1α (PGC1α), silent mating-type information regulation 2 homolog 3 (Sirt3) and manganese superoxide dismutase (MnSOD) were all decreased in the skeletal muscle of diabetic patients. Male rats with diabetes were also treated with the vehicle or with Celastrol at 1, 3 and 6 mg/kg/day for 8 weeks. The administration of Celastrol at 3 and 6 mg/kg attenuated the deterioration of skeletal muscle, as shown by histological analysis, decreased the malondialdehyde (MDA) level and increased the glutathione (GSH) level assayed by enzyme-linked immunosorbent assay (ELISA) method. It also enhanced the enzyme activity and increased the expression of MnSOD, and increased the AMPK phosphorylation level, as well as PGC1α and Sirt3 expression. The findings of our study suggest that the expression of AMPK, PGC1α, Sirt3 and MnSOD are decreased in the skeletal muscle of diabetic patients. Celastrol exerted antioxidant effects on skeletal muscle partly by regulating the AMPK-PGC1α-Sirt3 signaling pathway. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)