| In vitro: |
| J Agric Food Chem. 2014 Nov 12;62(45):11005-15. | | Degradation of curcuminoids by in vitro pure culture fermentation.[Pubmed: 25317751] | Colonic bacteria may mediate the transformation of curcuminoids, but studies of this metabolism are limited.
METHODS AND RESULTS:
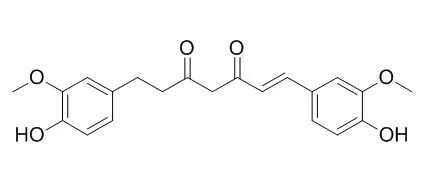
Here, the metabolism of curcuminoids by Escherichia fergusonii (ATCC 35469) and two Escherichia coli strains (ATCC 8739 and DH10B) was examined in modified medium for colon bacteria (mMCB) with or without pig cecal fluid. LC-MS analysis showed that 16-37% of curcumin, 6-16% of demethoxycurcumin (DMC) and 7-15% of bis-demethoxycurcumin (Bis-DMC), and 7-15% of bis-demethoxycurcumin (Bis-DMC) were converted following 36 h of fermentation, with the amount of curcuminoids degraded varying depending on the bacterial strain and medium used. Three metabolites (Dihydrocurcumin (DHC), tetrahydrocurcumin (THC), and ferulic acid (FA)) were found in fermentation cultures with all strains used. In addition, a compound with m/z [M - H](-) 470 was found and identified to be a curcumin adduct (curcumin-l-cysteine), using accurate mass FT-ICR-MS.
CONCLUSIONS:
This study provides insights into the bacterial metabolism of curcuminoids. | | Proc Natl Acad Sci U S A. 2011 Apr 19;108(16):6615-20. | | Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism.[Pubmed: 21467222] | Polyphenol curcumin, a yellow pigment, derived from the rhizomes of a plant (Curcuma longa Linn) is a natural antioxidant exhibiting a variety of pharmacological activities and therapeutic properties. It has long been used as a traditional medicine and as a preservative and coloring agent in foods.
METHODS AND RESULTS:
Here, curcumin-converting microorganisms were isolated from human feces, the one exhibiting the highest activity being identified as Escherichia coli. We are thus unique in discovering that E. coli was able to act on curcumin. The curcumin-converting enzyme was purified from E. coli and characterized. The native enzyme had a molecular mass of about 82 kDa and consisted of two identical subunits. The enzyme has a narrow substrate spectrum, preferentially acting on curcumin. The microbial metabolism of curcumin by the purified enzyme was found to comprise a two-step reduction, curcumin being converted NADPH-dependently into an intermediate product, Dihydrocurcumin, and then the end product, tetrahydrocurcumin.
CONCLUSIONS:
We named this enzyme "NADPH-dependent curcumin/Dihydrocurcumin reductase" (CurA). The gene (curA) encoding this enzyme was also identified. A homology search with the BLAST program revealed that a unique enzyme involved in curcumin metabolism belongs to the medium-chain dehydrogenase/reductase superfamily. | | Biomed Pharmacother . 2018 Jul;103:1327-1336. | | Dihydrocurcumin ameliorates the lipid accumulation, oxidative stress and insulin resistance in oleic acid-induced L02 and HepG2 cells[Pubmed: 29864915] | | Abstract
Aims: Curcumin is a polyphenol compound with many pharmacological activities including antioxidant, lipid-loweing and liver protective. Dihydrocurcumin (DHC) is one of the major metabolites of curcumin. So far, the pharmacological activity of DHC has not been reported. Here, we evaluate the effects of DHC on oleic acid (OA)-induced lipid accumulation, oxidative stress and insulin resistance and the underlying mechanism in L02 and HepG2 cells.
Main methods: OA-induced L02 and HepG2 cells were used as the in vitro model of nonalcoholic fatty liver disease (NAFLD). Lipid accumulation, oxidative stress, glucose uptake and cell inflammation were evaluated by cellular biochemical assay, respectively. Signaling pathways involved in lipid metabolism including peroxisome proliferator activated receptor-α (PPARα), the sterol regulatory element binding protein-1C (SREBP-1C) and patatin-like phospholipase domain containing 3 (PNPLA3), glucose uptake including phosphatidylinositol 3-kinase (PI3K) and phosphorylated serine-threonine protein kinase (pAKT), and oxidative stress including nuclear factor E2-related factor 2 (Nrf2), cytochrome P450 4A (CYP4A) and 2E1 (CYP2E1) were investigated by western blotting and RT-qPCR, respectively.
Key findings: DHC decreased the levels of cellular triglycerides (TG) by regulating the mRNA and protein expression levels of SREBP-1C, PNPLA3 and PPARα. At the same time, DHC improved the hepatocellular glucose uptake by increasing the protein expression levels of pAKT and PI3K. Furthermore, DHC reduced the levels of cellular NO and ROS via Nrf2 signaling pathways.
Significance: The present study firstly revealed that DHC ameliorated OA-induced steatosis through regulating the lipid metabolism, oxidative stress and insulin resistance in HepG2 and L02 cells.
Keywords: Dihydrocurcumin; Insulin resistance; Lipid metabolism; NAFLD; Oxidative stress. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)