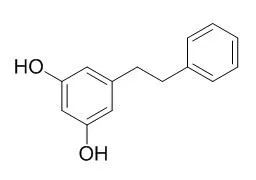
| Description: |
Dihydropinosylvinin is an phytoalexin, it shows antifungal activity against Cladosporium cladosporioides, Botryodiplodia theobromae, Aspergillus niger and Penicillium schlerotgenum, it also exhibits strong antibacterial activity against Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli.Dihydropinosylvin and batatasin IV can inhibit the germination of seeds of and root elongation in young seedlings of Sorghum bicolor. |
| In vitro: |
| Phytochemistry, 1989, 28(10):2621-5. | | Induction of pal activity and dihydrostilbene phytoalexins in Dioscorea alata and their plant growth inhibitory properties[Reference: WebLink] |
METHODS AND RESULTS:
Dihydropinosylvin, batatasin IV, demethylbatatasin IV and batatasin III were found in the water yam ( Dioscorea alata ) which had been inoculated with Botryodiplodia theobromae or treated with mercuric chloride.
Following induction, transient increases were observed in the first three compounds and this was preceded by a transient increase in the activity of phenylalanine ammonia lyase but not tyrosine ammonia lyase activity.
CONCLUSIONS:
In mercuric chloride treated tubers an increase in polyphenol oxidase was also observed.
The dormancy inducing compounds Dihydropinosylvin and batatasin IV were also found to inhibit the germination of seeds of and root elongation in young seedlings of Sorghum bicolor . By comparison, demethylbatatasin IV was not inhibitory. | | Phytochemistry, 1987, 26(12): 3187-9. | | Dihydrostilbene phytoalexins from Dioscorea rotundata.[Reference: WebLink] |
METHODS AND RESULTS:
2′,3-Dihydroxy-5-methoxybibenzyl (Batatasin IV), its demethyl derivative and 3,5-dihydroxybibenzyl (Dihydropinosylvin) were isolated only from flesh of Dioscorea rotundata infected with Botryodiplodia theobromae and may therefore be considered phytoalexins. These compounds were found to be antifungal using bioassays with Cladosporium cladosporioides, Botryodiplodia theobromae, Aspergillus niger and Penicillium schlerotgenum .
CONCLUSIONS:
Dihydro-pinosylvinin also exhibited strong antibacterial activity against Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli . | | Phytochemistry. 2004 Jan;65(1):99-106. | | Dihydrophenanthrenes and other antifungal stilbenoids from Stemona cf. pierrei.[Pubmed: 14697275] |
METHODS AND RESULTS:
Three new dihydrophenanthrenes, stemanthrenes A-C, along with the new dihydrostilbene stilbostemin G were isolated and identified from the underground parts of Stemona cf. pierrei together with the known pinosylvin, 4'-methylpinosylvin, Dihydropinosylvin, stilbostemins B, D, and E as well as the pyrrolo[1,2-a]azepine alkaloids protostemonine and stemonine.
The structures of all new stilbenoids, elucidated by NMR analyses, showed a common substitution pattern for aromatic ring A and characteristic C-methylations for ring B. The trivial name racemosol, previously reported for S. collinsae, was renamed to stemanthrene D due to its priority for another compound. Bioautographic tests on TLC plates with Cladosporium herbarum displayed high antifungal activity for compounds with an unsubstituted aromatic ring A, e.g. pinosylvin, but only weak effects for the higher substituted stilbostemin G and stemanthrenes A-C.
CONCLUSIONS:
Similar results were obtained by germ tube inhibition of five microfungi using 2-fold serial broth dilutions determined by a microplate reader. Because of weak inhibition and chemical instability of stemanthrenes, no EC(50) and EC(90) values could be calculated. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)