| In vitro: |
| Acta Chimica Sinica, 1988, 46(5): 483-8. | | Studies on the Constituents of Schisandra Henryi Ⅴ. The Structures of Wulignan A1, A2, Epiwulignan A1 and Epischisandrone)[Reference: WebLink] |
METHODS AND RESULTS:
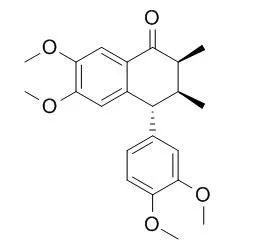
Four new lignan compounds named wulignan A1 (4), A2(5), epiwulignan A1 (6) and epischisandrone(7), and three known lignan compounds, enshicine(1), epienshicine(2) and schisandrone(3), were isolated from the fruits of Schisandra henryi Clarke collected in the district of Yibin, Sichuan Province. In anticancer screening all the four new compounds exhibit the activity against leukemia P-388 in vitro. Wulignan A_1(4), m. p. 195—197℃, [α]_D~(140-38.9°(CHCl_3), wulignan A_2(5), m. p. 235-237℃, [α]_D~(14)-61.2°(CH_3OH), epiwulignan A_1(6), m. p. 185-187℃, [α]_D~(14)+13.1°(CHCl_3), and epischisandrone (7), m. p. 182-183.5℃, [α]_D~(14)+5.5°(CHCl_3), display the features of tetralone lignan in their UV, IR, ~1H NMR (Table 1) and MS spectra.
CONCLUSIONS:
Besides the spectral analysis and NOE measurements, the structure assignments including absolute configurations mainly rely on the chemical transformation of 5, 6 and 7 into known compound, (+)-dimethylguaiacine(6b), and of 4 into known compound, (-)-dimethylisoguaiacine(4b). In addition, the following new compounds, (+)-isootobaphenol (2a), (+)-demethyleneisootobaphenol (2b), Dimethylwulignan A1 (4a) and dimethylepiwulignan A1 (6a) were prepared during the course of the structural elucidation. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)