| In vitro: |
| Journal of Advanced Oxidation Technologies, 2016,19(1):125-133. | | Fenton’s Oxidation Kinetics, Pathway, and Toxicity Evaluation of Diethyl Phthalate in Aqueous Solution.[Reference: WebLink] | A comprehensive study of the chemical oxidation degradation of diethyl phthalate (DEP) was conducted through Fenton processes.
METHODS AND RESULTS:
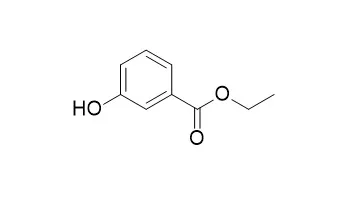
Effects of various operating parameters that considerably affect DEP decomposition were investigated, including solution pH, H2O2, Fe2+, and DEP concentration. The removal efficiency of DEP achieved 98% under reaction conditions of pH value of 3.0, concentration of 0.3 mM of Fe2+, and 6.0 mM of H2O2 after 170 min. In general, DEP degradation in Fenton process was found to occur in two stages, with an extremely fast stage and then a slow one, as a result of change of H2O2 and Fe2+ initial concentration. Based on the pseudo-steady-state hypothesis of hydroxyl radical formed by the Fenton reaction, a kinetic model for DEP degradation has been proposed which describes the effect of decomposition byproducts on the oxidation reaction. The experiment results also were in good agreement with Behnajady-Modirshahla-Ghanbery (BMG) kinetic model. During the degradation process, seven degradation intermediates of DEP were detected out by means of GC/MS, including ethyl 2-hydroxybenzoate, Ethyl 3-hydroxybenzoate, phthalic anhydride, benzoic acid ethyl ester, malonic acid, oxalic acid, and acetic acid. Probable degradation pathway of DEP by the Fenton reaction was also proposed. Inhibitory effects of DEP and intermediate products were investigated in aqueous solution with Photobacterium phosphoreum.
CONCLUSIONS:
Findings indicated that the solution was not completely detoxified even if DEP completely disappeared, further post-treatment was recommended. All these observations have significant potential applications and require further investigation. | | International journal of food microbiology, 2009, 131(2-3):178-182. | | In vitro antimycotic activity of a Williopsis saturnus killer protein against food spoilage yeasts.[Reference: WebLink] |
METHODS AND RESULTS:
The in vitro antimycotic activity of a purified killer protein (KT4561) secreted by a strain of Williopsis saturnus was tested against 310 yeast strains belonging to 21 food spoilage species of 14 genera (Candida, Debaryomyces, Dekkera, Hanseniaspora, Issatchenkia, Kazachstania, Kluyveromyces, Pichia, Rhodotorula, Saccharomyces, Schizosaccharomyces, Torulaspora, Yarrowia and Zygosaccharomyces). Minimum inhibitory concentration (MIC) determinations showed that over 65% of the target strains were susceptible to concentrations ≤ 32 µg/ml of KT4561. Three conventional food-grade antimicrobial agents were used as controls: 41, 33 and 40% of the target strains were sensitive to ≤ 512 mg/ml of Ethyl 3-hydroxybenzoate (E214), potassium sorbate (E202) or potassium metabisulphite (E224), respectively. The susceptibility of food spoilage yeasts towards KT4561, E214, E202 and E224 was species- and strain-dependent. In most cases KT4561 exhibited MIC values several orders of magnitude lower (100 to 100,000 times) than those observed for E214, E202 and E224. With only a few exceptions, the activity of KT4561 was pH-, ethanol-, glucose- and NaCl-independent.
CONCLUSIONS:
The present study demonstrates the potential of this yeast killer protein as a novel and natural control agent against food spoilage yeasts.
|
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)