| In vitro: |
| Journal of ethnopharmacology, 2017, 210:287. | | Network pharmacology analysis of the anti-cancer pharmacological mechanisms of Ganoderma lucidum extract with experimental support using Hepa1-6-bearing C57 BL/6 mice.[Reference: WebLink] | Ganoderma lucidum (GL) is an oriental medical fungus, which was used to prevent and treat many diseases. Previously, the effective components of Ganoderma lucidum extract (GLE) were extracted from two kinds of GL, Ganoderma lucidum (Leyss. Ex Fr.) Karst. and Ganoderma sinense Zhao, Xu et Zhang, which have been used for adjuvant anti-cancer clinical therapy for more than 20 years. However, its concrete active components and its regulation mechanisms on tumor are unclear. In this study, we aimed to identify the main active compounds from GLE and to investigate its anti-cancer mechanisms via drug-target biological network construction and prediction.
METHODS AND RESULTS:
The main active compounds of GLE were identified by HPLC, EI-MS and NMR, and the compounds related targets were predicted using docking program. To investigate the functions of GL holistically, the whole active components of GL and related targets were predicted based on four public databases. Subsequently, the compound-target network and component-target network were constructed respectively, and they were overlapped to detect the kernel potential targets in both networks. Furthermore, the qRT-PCR was used to validate the expression levels of target genes in GLE treated Hepa1-6-bearing C57 BL/6 mice. In our work, 12 active compounds of GLE were identified, including Ganoderic acid A, Ganoderenic acid A, Ganoderic acid B, Ganoderic acid H, Ganoderic acid C2, ganoderenic acid D, Ganoderic acid D, Ganoderenic acid G, Ganoderic acid Y, Kaemferol, Genistein and Ergosterol. Using the docking program, 20 targets were mapped to 12 compounds of GLE. Furthermore, 122 effective active components of GL and 116 targets were holistically predicted using public databases. Compare with the compound-target network and component-target network, 6 kernel targets were screened, including AR, CHRM2, ESR1, NR3C1, NR3C2 and PGR, which was considered as potential markers and might play important roles in the process of GLE treatment. GLE effectively inhibited tumor growth in Hepa1-6-bearing C57 BL/6 mice. Moreover, qRT-PCR data demonstrated the expression levels of NR3C1, NR3C2 and AR are up-regulated and PGR, ESR1 and CHRM2 are down-regulated.
CONCLUSIONS:
The results indicated that these 6 kernel target genes might act as potential markers to evaluate the curative effect of GLE treatment in tumor. The combined data provide preliminary study of the pharmacological mechanisms of GLE. GLE may be a promising potential therapeutic and chemopreventative candidate for anti-cancer. |
|


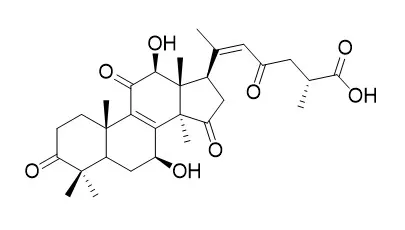



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)