| Cell Research: |
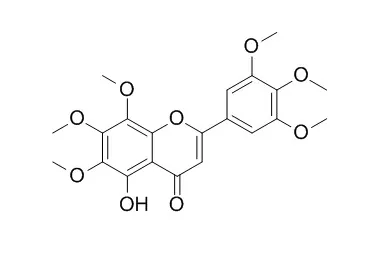
| Journal of Agricultural & Food Chemistry, 2013, 61(39):9453-9463. | | Neurotrophic Action of 5-Hydroxylated Polymethoxyflavones: 5-Demethylnobiletin and Gardenin A Stimulate Neuritogenesis in PC12 Cells.[Reference: WebLink] | Polymethoxyflavones (PMFs) exhibit a broad spectrum of biological properties, including anticancer, antiatherogenic, and neuroprotective effects. The aim of this study is to investigate the neurotrophic effects of 5-demethylnobiletin, a hydroxylated PMF found in citrus plants, and Gardenin A, a synthetic PMF analogue, on neurite outgrowth and neuronal differentiation in PC12 cells.
METHODS AND RESULTS:
The results of this study showed that 5-demethylnobiletin and Gardenin A (10–20 μM) potently induce neurite outgrowth in PC12 cells, accompanied by the expression of neuronal differentiation and synapse formation marker proteins, growth-associated protein-43 (GAP-43), and synaptophysin. We observed that the addition of K252a, a TrKA antagonist, significantly inhibited NGF-induced neurite outgrowth in PC12 cells, but 5-demethylnobiletin- or Gardenin A-induced neurite outgrowth was not affected. Treatment with 5-demethylnobiletin and Gardenin A markedly induced the phosphorylation of both cyclic AMP response element-binding protein (CREB) and CRE-mediated transcription, which was suppressed through the administration of the inhibitor 2-naphthol AS-E phosphate (KG-501) or using CREB siRNA. Furthermore, our results showed that MAPK/ERK kinase 1/2 (MEK1/2), protein kinase A (PKA), and protein kinase C (PKC) inhibitors blocked the CRE transcription activity and neurite outgrowth induced through 5-demethylnobiletin or Gardenin A. Consistently, increased ERK phosphorylation and PKA and PKC activities were observed in PC12 cells treated with 5-demethylnobiletin or Gardenin A.
CONCLUSIONS:
These results reveal for the first time that 5-demethylnobiletin and Gardenin A promote neuritogenesis through the activation of MAPK/ERK-, PKC-, and PKA-dependent, but not TrkA-dependent, CREB signaling pathways in PC12 cells. |
|
| Animal Research: |
| Chemico Biological Interactions, 2017, 269:9-17. | | Antihyperlipidemic and hepatoprotective effects of Gardenin A in cellular and high fat diet fed rodent models.[Reference: WebLink] | The gum of Gardenia resinifera Roth., is one of the important drugs used in the Indian system of medicine and a source of unique polymethoxylated flavones. This study was aimed to evaluate the antihyperlipidemic and anti-NAFLD effects of Gardenin A (Gar-A) from G. resinifera gum using in vitro and in vivo models. Gar-A was isolated from G. resinifera gum and was identified on the basis of the physical and spectral data.
METHODS AND RESULTS:
Toxicity of Gar-A to HepG2 cells was evaluated using MTT assay. The ability of Gar-A to reduce steatosis was assessed using oleate-palmitate induced HepG2 cell lines by estimating the lipid levels by ORO staining and by estimating the intracellular triglyceride content. Effect of Gar-A on amelioration of lipotoxicity was measured by estimating the LDH levels. The doses for in vivo experiments were fixed by Irwin test, between 50 and 100 mg/kg concentrations, through oral route. The acute antihyperlipidemic effect of Gar-A was assessed in Triton WR–1339 induced hyperlipidemic animals. The chronic antihyperlipidemic and anti-NAFLD effects of Gar-A were evaluated in HFD fed rats. In vitro experiments with HepG2 cell line indicated that the cells treated with Gar-A did not show any significant reduction in the viability up to 70 μg/mL concentration. Steatotic HepG2 cells treated with Gar-A showed a significant reduction in lipid accumulation at 2.5–10 μg/mL concentrations. In triton induced hyperlipidemic rats, the treatment significantly reduced the lipid levels at the synthesis phase.
CONCLUSIONS:
The treatment with Gar-A to the HFD fed animals significantly lowered the steatosis and transaminase levels. The other biochemical parameters such as TC, TG, LDL-c, ALP and ACP were also decreased significantly. Treatment with Gar-A significantly lowered the hyperlipidemia and fat accumulation in the liver; detailed molecular investigations are necessary to establish the antihyperlipidemic and hepatoprotective potentials of Gar-A. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)