| Kinase Assay: |
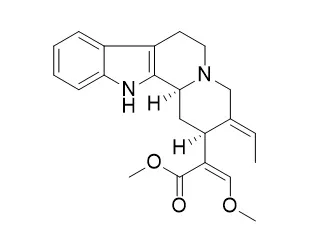
| Eur J Pharmacol. 2011 Dec 5;671(1-3):79-86. | | Geissoschizine methyl ether has third-generation antipsychotic-like actions at the dopamine and serotonin receptors.[Pubmed: 21951966 ] |
METHODS AND RESULTS:
In the present assay, the efficacy and potency of known ligands of serotonin 5-HT(1A), 5-HT(2A), 5-HT(2C), 5-HT(7) and dopamine D(2L) receptors were comparable to those found in previous studies using a variety of readouts. The developed assay was also able to reproduce the partial agonist activity, the low intrinsic activity and the selective activation of aripiprazole at the dopamine D(2L) receptors. Under identical experimental conditions, Geissoschizine methyl ether (GM), a plant indole alkaloid, behaved as a partial agonist at the serotonin 5-HT(1A) receptor, a partial agonist/antagonist at the dopamine D(2L) receptor and an antagonist at the serotonin 5-HT(2A), 5-HT(2C) and 5-HT(7) receptors. Interestingly, GM showed a relatively low intrinsic activity and evoked a partial activation response in a subset of cells expressing the dopamine D(2L) receptor; both of these effects were similarly observed for aripiprazole.
CONCLUSIONS:
Although GM is far less potent at the dopamine receptor than aripiprazole at dopamine D(2L) receptors (EC(50)=4.4 μM for GM vs. EC(50)=56 nM for aripiprazole), GM and GM derivatives may comprise a new set of candidates for atypical antipsychotics. | | Nat Prod Res. 2012;26(1):22-8. | | Geissoschizine methyl ether, a corynanthean-type indole alkaloid from Uncaria rhynchophylla as a potential acetylcholinesterase inhibitor.[Pubmed: 21714741 ] |
METHODS AND RESULTS:
Geissoschizine methyl ether (1), a newly discovered strong acetylcholinesterase (AChE) inhibitor, along with six weakly active alkaloids, vallesiachotamine (2), hisuteine (3), hirsutine (4), isorhynchophylline (5), cisocorynoxeine (6) and corynoxeine (7) have been isolated from Uncaria rhynchophylla.
Geissoschizine methyl ether (1) inhibited 50% of AChE activity at concentrations of 3.7 ± 0.3 µg mL(-1) while the IC(50) value of physostigmine as a standard was 0.013 ± 0.002 µg mL(-1).
CONCLUSIONS:
The mode of AChE inhibition by 1 was reversible and non-competitive. In addition, molecular modelling was performed to explore the binding mode of inhibitor 1 at the active site of AChE. |
|
| Cell Research: |
| J Ethnopharmacol. 2016 Jul 1;187:249-58. | | Geissoschizine methyl ether protects oxidative stress-mediated cytotoxicity in neurons through the 'Neuronal Warburg Effect'.[Pubmed: 27114061] | The rate of production of reactive oxygen species (ROS) is determined by mitochondrial metabolic rate. In turn, excessive ROS damage mitochondrial function, which is linked to aging and neurodegenerative conditions. One possible path to prevent oxidative stress could be achieved by reducing mitochondrial respiration in favor of less efficient ATP production via glycolysis. Such a shift in energy metabolism is known as the 'Warburg effect'. Geissoschizine methyl ether (GM) is one of the active components responsible for the psychotropic effects of Yokukansan, an herbal preparation widely used in China and Japan.
GM protects neurons from glutamate-induced oxidative cytotoxicity through regulating mitochondrial function and suppressing ROS generation. We investigated the protective mechanism of GM against glutamate-induced oxidative stress in neuronal cells.
METHODS AND RESULTS:
The current study was performed on primary neurons and HT22 cells, a hippocampus neuronal cell line. Cell viability was measured by Calcein AM assay. H2DCFDA staining was used for intracellular ROS measurement. GSH level was measured using the GSH-Glo™ luminescence-based assay. Mitochondrial respiration and glycolysis were measured by the Seahorse Bioscience XFe 96 Extracellular Flux Analyzer. Protein levels were analyzed by western blot analysis.
GM prevented glutamate-induced cytotoxicity in an HT-22 neuronal cell line even with a 9-hour exposure delay. GM blocked glutamate-induced intracellular ROS accumulation through suppressing mitochondrial respiration. Further, we found that GM up-regulated glycolysis and the pentose-phosphate pathway, which is involved in the production of intracellular reducing agent, NADPH. In addition, GM protected primary cortical neurons from both glutamate and buthioninesulfoximine toxicity.
CONCLUSIONS:
GM prevents glutamate-induced oxidative damage through reducing mitochondrial respiration, which further suppresses ROS generation. In addition, GM up-regulates glycolysis which compensate for the energy depletion induced by mitochondrial respiration inhibition. Overall, our study is the first to report that GM protects neurons from oxidative toxicity by shifting energy metabolism from mitochondrial respiration to glycolysis. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)