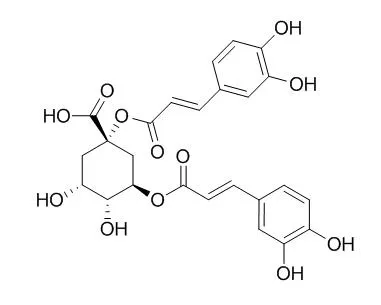
| In vitro: |
| Food Research International, 2009 , 42 (9) :1273-1280. | | Antioxidant activity of 1,3-dicaffeoylquinic acid isolated from Inula viscosa.[Reference: WebLink] | Inula viscosa is a perennial herbaceous plant used topically in folk medicine as an anti-scabies, anti-inflammatory, and wound-healing agent.
METHODS AND RESULTS:
We examined the antioxidant activity of the methanolic extract of I. viscosa. We isolated and identified several polyphenolic antioxidants from I. viscosa leaves and focused on 1,3-Dicaffeoylquinic acid (1,3-diCQA). Antioxidant activity was measured using ABTS and DPPH assays, which measure antioxidant activity. The concentrations of 1,3-diCQA required for the inhibition of oxidation were lower than those required by other known antioxidants. 1,3-diCQA inhibited oxidative damage caused by various factors, including FeSO4 and AAPH (2,2'-azobis(2-amidinopropane) dehydrochloride). Antioxidant activity can also be detected by the ability of a compound to scavenge reactive oxygen species (ROS). 1,3-diCQA was found to scavenge hydroxyl radical and superoxide radicals, as measured by electron spin resonance (ESR).
CONCLUSIONS:
These data demonstrate that 1,3-diCQA exhibits antioxidant properties, probably through the involvement of a direct scavenging effect on several free radicals. |
|
| In vivo: |
| Evid Based Complement Alternat Med. 2013;2013:612527. | | Interaction of Natural Dietary and Herbal Anionic Compounds and Flavonoids with Human Organic Anion Transporters 1 (SLC22A6), 3 (SLC22A8), and 4 (SLC22A11).[Pubmed: 23573138] | Active components of complementary/alternative medicines and natural supplements are often anionic compounds and flavonoids. As such, organic anion transporters (OATs) may play a key role in their pharmacokinetic and pharmacological profiles, and represent sites for adverse drug-drug interactions.
METHODS AND RESULTS:
Therefore, we assessed the inhibitory effects of nine natural products, including flavonoids (catechin and epicatechin), chlorogenic acids (1,3-Dicaffeoylquinic acid and 1,5-Dicaffeoylquinic acid), phenolic acids (ginkgolic acids (13 : 0), (15 : 1), and (17 : 1)), and the organic acids ursolic acid and 18 β -glycyrrhetinic acid, on the transport activity of the human OATs, hOAT1 (SLC22A6), hOAT3 (SLC22A8), and hOAT4 (SLC22A11). Four compounds, 1,3- and 1,5-Dicaffeoylquinic acid, ginkgolic acid (17 : 1), and 18 β -glycyrrhetinic acid, significantly inhibited hOAT1-mediated transport (50 μ M inhibitor versus 1 μ M substrate). Five compounds, 1,3- and 1,5-Dicaffeoylquinic acid, ginkgolic acids (15 : 1) and (17 : 1), and epicatechin, significantly inhibited hOAT3 transport under similar conditions. Only catechin inhibited hOAT4. Dose-dependency studies were conducted for 1,3-Dicaffeoylquinic acid and 18 β -glycyrrhetinic acid on hOAT1, and IC50 values were estimated as 1.2 ± 0.4 μ M and 2.7 ± 0.2 μ M, respectively.
CONCLUSIONS:
These data suggest that 1,3-Dicaffeoylquinic acid and 18 β -glycyrrhetinic acid may cause significant hOAT1-mediated DDIs in vivo; potential should be considered for safety issues during use and in future drug development. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)