| Kinase Assay: |
| Bioorg Med Chem Lett. 2010 Sep 15;20(18):5398-401 | | Prenylflavonoids from Glycyrrhiza uralensis and their protein tyrosine phosphatase-1B inhibitory activities.[Pubmed: 20724155 ] |
METHODS AND RESULTS:
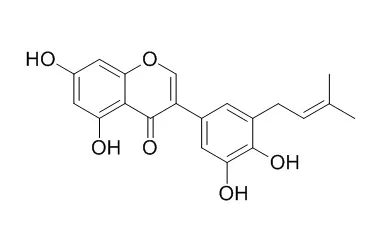
Two new 2-arylbenzofurans, glycybenzofuran (1) and cyclolicocoumarone (2), together with 10 known flavonoids including licocoumarone (3), Glycyrrhisoflavone (4), glisoflavone (5), cycloGlycyrrhisoflavone (6), isoliquiritigenin (7), licoflavone A (8), apigenin (9), isokaempferide (10), glycycoumarin (11), and isoglycycoumarin (12), were isolated from the roots of Glycyrrhiza uralensis and their structures were determined by extensive spectroscopic analyses. Compounds 1 and 5 showed significant protein tyrosine phosphatase-1B (PTP1B) inhibitory activity in vitro with the IC50 values of 25.5 and 27.9 microM, respectively. The structure-activity relationship indicated that the presence of prenyl group and ortho-hydroxy group is important for exhibiting the activity.
CONCLUSIONS:
Kinetic analysis indicated that compound 1 inhibits PTP1B by a competitive mode, whereas compound 5 by a mixed mode. | | Planta Med. 2005 Aug;71(8):785-7. | | Identification of tyrosinase inhibitors from Glycyrrhiza uralensis.[Pubmed: 16142649] | Tyrosinase is a key enzyme in the production of melanins.
METHODS AND RESULTS:
Phytochemical studies of a Glycyrrhiza uralensis extract were performed by measuring the tyrosinase and melanin synthesis inhibitory activity. Glycyrrhisoflavone and glyasperin C were identified as tyrosinase inhibitors for the first time. Glyasperin C showed a stronger tyrosinase inhibitory activity (IC (50) = 0.13 +/- 0.01 microg/mL) than glabridin (IC (50) = 0.25 +/- 0.01 microg/mL) and a moderate inhibition of melanin production (17.65 +/- 8.8 % at 5 microg/mL). Glycyrrhisoflavone showed a strong melanin synthesis inhibitory activity (63.73 +/- 6.8 % inhibition at 5 microg/mL).
CONCLUSIONS:
These results suggest that glyasperin C and Glycyrrhisoflavone could be promising candidates in the design of skin-whitening agents. | | Chem Pharm Bull (Tokyo). 1991 May;39(5):1238-43. | | Phenolic constituents of licorice. III. Structures of glicoricone and licofuranone, and inhibitory effects of licorice constituents on monoamine oxidase.[Pubmed: 1913999] | Two new phenolic compounds, glicoricone (3) and licofuranone (4), were isolated from a species of licorice brought from the northwestern region of China, and their structures were assigned.
METHODS AND RESULTS:
Among the twelve licorice constituents examined for the inhibition of monoamine oxidase (MAO), six compounds, 3, 4, genistein (6), licopyranocoumarin (7), licocoumarone (14) and Glycyrrhisoflavone (15), inhibited the enzyme with the IC50 (concentration required for 50% inhibition of the enzyme activity) values of 6.0 x 10(-5)-1.4 x 10(-4) M. Glycyrrhizin (1) also inhibited MAO with the IC50 value of 1.6 x 10(-4) M. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)