| Description: |
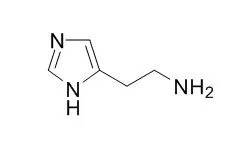
Histamine, an organic nitrogenous compound, is involved in local immune responses regulating physiological function in the gut and acting as a neurotransmitter for the brain, spinal cord, and uterus. It is a potent H1 and H2 receptor agonist. Histamine increases Nav1.8 expression in primary afferent neurons via H2 receptor-mediated pathway and thereby contributes to neuropathic pain, H2 receptor antagonists may potentially be used as analgesics for patients with neuropathic pain. |
| In vivo: |
| Acta Microbiol Immunol Hung. 2015 Mar;62(1):87-91. | | Modulation of ConA-induced inflammatory ascites by histamine - short communication.[Pubmed: 25823456] | The early phase of the ConA-induced inflammatory ascites was studied, with special reference to Histamine.
METHODS AND RESULTS:
Concanavalin A (ConA), a cell-surface binding lectin was injected i.p. (25 mg/kg bw) to mice. After 1 h the animals were killed, the ascitic fluid collected and measured. Other agents were injected s.c., 10 min before the ConA-challenge. Exogenous Histamine markedly inhibited the ConA-induced ascites. Release of endogenous vasoactive agents from the mast cells by Compound 48/80 had a similar, but slight effect. Cromolyn, a mast cell stabilizing agent, and chloropyramine, a Histamine H1 receptor antagonist was ineffective. Although Histamine increases endothelial permeability, it did not enhance the formation of ascitic fluid, on the contrary, it inhibited the ConA-induced ascites, presumably due to its known hypotonic effect.
CONCLUSIONS:
It is concluded that ConA-induced ascites is not mediated by mast cell Histamine. | | Eur J Immunol. 2015 Apr;45(4):1129-40. | | The histamine H4 -receptor (H4 R) regulates eosinophilic inflammation in ovalbumin-induced experimental allergic asthma in mice.[Pubmed: 25501767] | Via the Histamine H4 -receptor (H4 R), Histamine promotes the pathogenesis of experimental allergic asthma in mice. Application of H4 R antagonists during sensitization as well as during provocation reduces the severity of the disease. However, the specific cell types functionally expressing H4 R in experimental allergic asthma have not been well characterized in vivo.
METHODS AND RESULTS:
In this study, we identified the cell type(s) responsible for H4 R activity in experimental asthma and related physiological mechanisms. Using H4 R-deficient mice, we studied the role of H4 R in the sensitization and effector phase. DCs lacking H4 R expression during the in vitro sensitization reaction resulted in effector T cells unable to induce an entire eosinophilic inflammation in the lung upon adoptive transfer in vivo. Recipient mice lacking H4 R expression, which were adoptively transferred with H4 R(+/+) T cells polarized in the presence of H4 R(+/+) DCs, showed reduced signs of inflammation and ameliorated lung function. Here, we provide in vivo evidence that in experimental asthma in mice the H4 R specifically regulates activation of DCs during sensitization, while in the effector phase the H4 R is active in cells involved in the activation of eosinophils, and possibly other cells.
CONCLUSIONS:
A putative therapy targeting the H4 R may be an option for asthma patients developing IL-5-dependent eosinophilia. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)