| Cell Research: |
| Nat Prod Commun. 2011 May;6(5):575-9. | | Bioactivity-guided study of antiproliferative activities of Salvia extracts.[Pubmed: 21615011] | The cytotoxic activities of the n-hexane, chloroform and aqueous methanolic fractions prepared from the methanolic extract of the leaves of 23 Salvia taxa were studied for their cell growth-inhibitory activity against human cervix adenocarcinoma (HeLa), skin carcinoma (A431) and breast adenocarcinoma (MCF7) cells using the MTT assay.
METHODS AND RESULTS:
The n-hexane fractions of six Salvia taxa (S. hispanica, S. nemorosa, S. nemorosa 1. albiflora, S. pratensis, S. recognita and S. ringens) and the chloroform fraction ofS. officinalis 1. albiflora produced over 50% growth inhibition of the skin carcinoma cell line. None of the tested extracts showed substantial (above 50%) antiproliferative effects against HeLa and MCF7 cells. S. ringens was the most powerful among the studied Salvia species with a 61.8% cell growth inhibitory activity on A431 cells. In the case of S. ringens, other plant parts were also tested for antiproliferative effect, and the highest activities were recorded for the root extract. This was subjected to bioactivity-guided fractionation, which yielded four abietane diterpenes (royleanone, Horminone, 7-O-methyl-Horminone and 7-acetyl-Horminone), one triterpene (erythrodiol-3-acetate) and beta-sitosterol.
CONCLUSIONS:
Horminone, 7-acetyl-Horminone and erythrodiol-3-acetate displayed marked concentration-dependent antiproliferative effects, while royleanone and 7-O-methyl-Horminone produced weaker activities. |
|
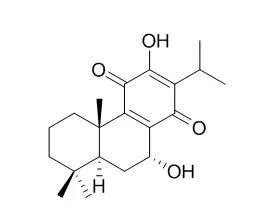
| Structure Identification: |
| Int. J. Quantum Chem.,2003, 93(6):411-21. | | Theoretical study of the structure and antimicrobial activity of horminone.[Reference: WebLink] |
METHODS AND RESULTS:
The structural and electronic parameters of the Horminone molecule, an abietan diterpene quinone, were studied by means of all-electron calculations using Hartree–Fock and density functional theory-based methods, as implemented in the Gaussian98 program. The 6-31G orbital basis sets were used for the C, H, O, and Mg atoms.
CONCLUSIONS:
The results allow the identification of the negative site of Horminone (HM) most favorable for its binding to the Mg2+ ion. The HM–Mg2+ complex is assumed to play a significant role in the antibacterial activity. First, it penetrates the membrane cell. Then, through its interaction with rRNA, it inhibits the protein synthesis in several types of bacteria. | | J Phys Chem A. 2006 Apr 6;110(13):4564-73. | | Theoretical study of the complexes of horminone with Mg2+ and Ca2+ ions and their relation with the bacteriostatic activity.[Pubmed: 16571064 ] |
METHODS AND RESULTS:
The coordination of the Horminone molecule with hydrated magnesium and calcium divalent ions was studied by means of the density functional theory. All-electron calculations were performed with the B3LYP/6-31G method. The first layer of the water molecules surrounding the metallic cations was included. It was found that the octahedral [Horminone(O(a)-O(d))-Mg-(H(2)O)(4)](2+) complex is more stable than [Mg(H(2)O)(6)](2+). That is, Horminone is able to displace two water units from the hexahydrated complex. This behavior does not occur for Ca(2+). Consistently, [Horminone(O(a)-O(d))-Mg-(H(2)O)(4)](2+) has a greater metal-ligand binding energy than [Horminone(O(a)-O(d))-Ca-(H(2)O)(4)](2+). The preference of Horminone by Mg(2+) is enlightened by these results. Moreover, its electronic structure, as shown by huge changes in the atomic populations, is strongly perturbed by Mg(2+). Indeed, Horminone, bonded to [Mg(H(2)O)(4)](2+), is able to cross the bacterial membrane cell. Once inside, [Horminone(O(a)-O(d))-Mg-(H(2)O)(4)](2+) binds to rRNA phosphate groups yielding [Horminone(O(a)-O(d))-Mg-(H(2)O)(PO(4)H(2))(PO(4)H(3))(2)](+).
CONCLUSIONS:
These results give insights into how Horminone may inhibit the initial steps of protein synthesis. The stability of the studied systems is accounted for in terms of the calculated structural and electronic properties: Mg-O and Ca-O bond lengths, charge transfers, and binding energies. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)