| In vitro: |
| J Agric Food Chem. 1999 Oct;47(10):4117-20. | | Physical stability of shikonin derivatives from the roots of Lithospermum erythrorhizon cultivated in Korea.[Pubmed: 10552776] |
METHODS AND RESULTS:
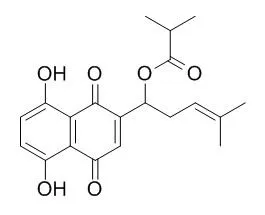
Five red shikonin pigments, deoxyshikonin, shikonin, acetylshikonin, Isobutylshikonin, and beta-hydroxyisovalerylshikonin, were isolated from the roots of Lithospermum erythrorhizon cultivated in Korea.
The purified pigments were red, purple, and blue at acidic, neutral, and alkaline pH values, respectively. Physical stability of the purified pigments against heat and light in an aqueous solution was examined for possible value-added food colorants. The thermal degradation reactions were carried out at pH 3.0 (50 mM glycine buffer) in 50% EtOH/H(2)O. Deoxyshikonin (t(1/2) = 14.6 h, 60 degrees C) and isobutylshikinin (t(1/2) = 19.3 h, 60 degrees C) are relatively less stable than other shikonin derivatives (t(1/2) = 40-50 h, 60 degrees C). Activation energies of thermal degradation of the isolated pigments were calculated.
The activation energy of deoxyshikonin was the highest (12.5 kcal mol(-)(1)) and that of beta-hydroxyisovalerylshikonin was the lowest (1.71 kcal mol(-)(1)) value.
CONCLUSIONS:
Light stabilities of the pigments were similar to each other in that the half-life values of photodegradation for 20000 lx light intensity were 4.2-5.1 h. | | Food Chemistry, 2008, 106(1):2-10. | | Antioxidants from a Chinese medicinal herb – Lithospermum erythrorhizon[Reference: WebLink] | Seven compounds, deoxyshikonin (1), β,β-dimethylacrylshikonin (2), Isobutylshikonin (3), shikonin (4), 5,8-dihydroxy-2-(1-methoxy-4-methyl-3-pentenyl)-1,4-naphthalenedione (5), β-sitosterol (6) and a mixture of two caffeic acid esters [7 (7a,7b)] were isolated from Lithospermum erythrorhizon Sieb et. Zucc. and identified by spectroscopic methods.
Among them, compound 5 was isolated from this plant species for the first time.
METHODS AND RESULTS:
The antioxidant activities of the seven compounds were compared and evaluated through Rancimat method, reducing power and radical scavenging activity. Results showed that, except compound 6, another 6 compounds all exhibited obvious antioxidant activities against four different methods. Compounds 4 and 7 exerted much more potent antioxidant effects on retarding the lard oxidation than that of BHT and both were found to exhibit strong reducing power. Their antioxidant activities, assessed by Rancimat method and reducing power, decreased in the following order, respectively: compound 7 > 4 > BHT > 2 > 3 > 5 > 1 > 6 and compound 7 > BHT > 4 > 2 approximately 3 approximately 5 > 1> 6. In addition, compounds 1-5 all exerted very good radical scavenging activities toward ABTS+ but showed moderate inhibition of DPPH·, while compound 7 presented as a powerful radical scavenger against both ABTS·+ and DPPH·.
CONCLUSIONS:
Thus, our results suggested that L. erythrorhizon could be a promising rich source of natural antioxidants. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)