| In vitro: |
| Phytomedicine. 2016 Sep 15;23(10):1005-11. | | Acetylcholine esterase inhibitors and melanin synthesis inhibitors from Salvia officinalis.[Pubmed: 27444345] | Salvia officinalis is a traditionally used herb with a wide range of medicinal applications. Many phytoconstituents have been isolated from S. officinalis, mainly phenolic diterpenes, which possess many biological activities. This study aimed to evaluate the ability of the phenolic diterpenes of S. officinalis to inhibit acetylcholine esterase (AChE) as well as their ability to inhibit melanin biosynthesis in B16 melanoma cells.
METHODS AND RESULTS:
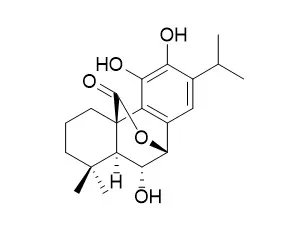
The phenolic diterpenes isolated from the aerial parts of S. officinalis were tested for their effect on melanin biosynthesis in B16 melanoma cell lines. They were also tested for their ability to inhibit AChE using Ellman's method. Moreover, a molecular docking experiment was used to investigate the binding affinity of the isolated phenolic diterpenes to the amino acid residues at the active sites of AChE. Seven phenolic diterpenes-sageone, 12-methylcarnosol, carnosol, 7b-methoxyrosmanol, 7a-methoxyrosmanol, Isorosmanol and epirosmanol-were isolated from the methanolic extract of the aerial parts of S. officinalis. Isorosmanol showed a melanin-inhibiting activity as potent as that of arbutin. Compounds 7a-methoxyrosmanol and Isorosmanol inhibited AChE activity by 50% and 65%, respectively, at a concentration of 500 μM.
CONCLUSIONS:
The results suggest that Isorosmanol is a promising natural compound for further studies on development of new medications which might be useful in ageing disorders such as the declining of cognitive functions and hyperpigmentation. | | J Agric Food Chem. 2002 Mar 27;50(7):1845-51. | | Antioxidant activity of chemical components from sage (Salvia officinalis L.) and thyme (Thymus vulgaris L.) measured by the oil stability index method.[Pubmed: 11902922] |
METHODS AND RESULTS:
A new abietane diterpenoid, 12-O-methyl carnosol (2), was isolated from the leaves of sage (Salvia officinalis L.), together with 11 abietane diterpenoids, 3 apianane terpenoids, 1 anthraquinone, and 8 flavonoids.
Antioxidant activity of these compounds along with 4 flavonoids isolated from thyme (Thymus vulgaris L.) was evaluated by the oil stability index method using a model substrate oil including methyl linoleate in silicone oil at 90 degrees C. Carnosol, rosmanol, epirosmanol, Isorosmanol, galdosol, and carnosic acid exhibited remarkably strong activity, which was comparable to that of alpha-tocopherol. The activity of miltirone, atuntzensin A, luteolin, 7-O-methyl luteolin, and eupafolin was comparable to that of butylated hydroxytoluene.
CONCLUSIONS:
The activity of these compounds was mainly due to the presence of ortho-dihydroxy groups. The 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity of these compounds showed the similar result. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)