| J Nat Prod. 2015 Mar 27;78(3):530-3. |
| Isolation and stereospecific synthesis of janolusimide B from a New Zealand collection of the bryozoan Bugula flabellata.[Pubmed: 25494238] |
METHODS AND RESULTS:
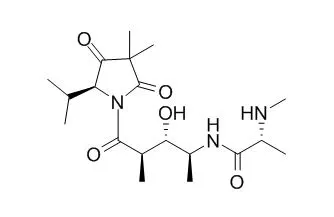
NMR-directed screening of New Zealand marine organisms has led to the isolation of the modified tripeptide Janolusimide B from the common invasive bryozoan Bugula flabellata. The structure was established by NMR and MS analysis, degradative hydrolysis and derivatization, and stereoselective fragment synthesis.
CONCLUSIONS:
The bryozoan natural product is an N-methyl analogue of Janolusimide, previously reported from the Mediterranean nudibranch Janolus cristatus, a species known to prey upon bryozoa. |
| Tetrahedron Letters,2000,41(21):3979–3982. |
| Stereochemistry and total synthesis of janolusimide, a tripeptide marine toxin[Reference: WebLink] |
METHODS AND RESULTS:
The stereochemistry of Janolusimide, a lipophilic tripeptide marine toxin, has been fully elucidated by stereoselective synthesis of the lactam component (5S)-3,3′-dimethyl-5-isopropylpyrrolidin-2,4-dione. The peptide was then synthesized in 13 steps and in 0.8% total yield. |
| Tetrahedron: Asymmetry,1999,10(10):1851–1854. |
| Stereoselective synthesis of 4-amino-3-hydroxy-2-methylpentanoic acids: stereochemistry of the amino acid occurring in the marine toxin janolusimide[Reference: WebLink] |
METHODS AND RESULTS:
Four diastereomers of 4-amino-3-hydroxy-2-methylpentanoic acid, an amino acid constituent of the hexapeptide portion of the antitumor antibiotic bleomycin A2, have been stereoselectively synthesized by crotylboration of N-Boc-l-alaninal.
CONCLUSIONS:
The synthesis allowed the assignment of the stereochemistry as 2R,3S,4S to the 4-amino-3-hydroxy-2-methylpentanoic acid occurring in the tripeptide marine toxin Janolusimide. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)