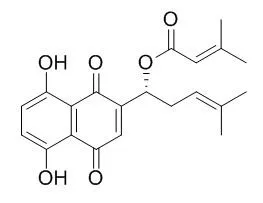
| Description: |
Dimethylacrylshikonin is a promising agent for developing an improved strategy for radiotherapy against tumors, it inhibits the proliferation of MCF-7 cells in vitro by inducing apoptosis through the downregulation of Bcl-2, upregulation of Bax and partial inactivation of the NF-κB pathway. Dimethylacrylshikonin inhibits agonist-induced relaxation at lower concentrations and induces vasocontraction at higher concentrations. |
| Targets: |
ROS | ERK | NF-kB | Bcl-2/Bax | Caspase | PARP | MEK | p38MAPK | Calcium Channel |
| In vitro: |
| Oncol Lett. 2014 Jun;7(6):1812-1818. | | β,β-Dimethylacrylshikonin sensitizes human colon cancer cells to ionizing radiation through the upregulation of reactive oxygen species.[Pubmed: 24932238] | Shikonin, a naphthoquinone derivative, has been shown to possess antitumor activity.
METHODS AND RESULTS:
In the present study, the effects of shikonin and its analog, β,β-Dimethylacrylshikonin, were investigated as radiosensitizers on the human colon cancer cell line, HCT-116. Shikonin and, to a greater extent, its analog-induced apoptosis of HCT-116 cells further synergistically potentiated the induction of apoptosis when combined with ionizing radiation (IR) treatment. Shikonins also stimulated an increase in reactive oxygen species (ROS) production and IR-induced DNA damage. Pre-treatment with the ROS scavenger, N-acetylcysteine, suppressed the enhancement of IR-induced DNA damage and apoptosis stimulated by shikonins, indicating that shikonins exert their radiosensitizing effects through ROS upregulation. The radiosensitizing effect of shikonins was also examined in vivo using the xenograft mouse model. Consistent with the in vitro results, injection of β,β-Dimethylacrylshikonin combined with IR treatment significantly suppressed tumor growth of the HCT-116 xenograft.
CONCLUSIONS:
Taken together, the results show that β,β-Dimethylacrylshikonin is a promising agent for developing an improved strategy for radiotherapy against tumors. | | Phytother Res. 2012 May;26(5):764-71. | | Inhibitory effects of β,β-dimethylacrylshikonin on hepatocellular carcinoma in vitro and in vivo.[Pubmed: 22109831] | Dimethylacrylshikonin is one of the most abundant naphthoquinones in the root extracts of Lithospermum erythrorhizon Sieb. et Zucc. (Boraginaceae), which have been reported to have antitumor effects.
METHODS AND RESULTS:
This study evaluated the antiproliferative activity of Dimethylacrylshikonin on human hepatocellular carcinoma (HCC) cells both in vitro and in vivo. In vitro, the MTT assay showed that Dimethylacrylshikonin inhibited the proliferation of SMMC-7721 cells in both dose- and time-dependent manners with its 50% inhibitory concentration (IC(50) ) at 48 h being 15.01 ± 0.76 µg/mL. Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling (TUNEL) and Hoechst staining detected the characteristics of cell apoptosis in Dimethylacrylshikonin-treated cells and the apoptotic rates of treated groups were increased in a dose-dependent manner. Flow cytometric analysis revealed that Dimethylacrylshikonin could block the cell cycle arrest at G2 phase. Furthermore, Dimethylacrylshikonin down-regulated the mRNA and protein expression of Bcl-2 but up-regulated that of Bax. The cleaved caspase-3 protein was also detected in treated cells. The experiment in vivo showed that Dimethylacrylshikonin significantly suppressed the growth of H(22) transplantable hepatoma, and induced the activation of caspase-3 determined by immunohistochemistry.
CONCLUSIONS:
The results indicate that Dimethylacrylshikonin has significant antitumor effects on hepatocellular carcinoma both in vitro and in vivo. | | Planta Med. 2004 Jan;70(1):23-8. | | Impairment of vascular function of rat thoracic aorta in an endothelium-dependent manner by shikonin/alkannin and derivatives isolated from roots of Macrotomia euchroma.[Pubmed: 14765288 ] |
METHODS AND RESULTS:
The effects of a naphthoquinone analogue, shikonin/alkannin (SA) and derivatives (acetylshikonin and beta,beta-Dimethylacrylshikonin), on vascular reactivity were studied with isolated rat aortic rings. At lower concentrations, SA and its derivatives concentration-dependently inhibit the agonist-induced (acetylcholine and histamine) relaxation in PE precontracted aorta in an endothelium-dependent manner with IC (50) values ranging from 0.2 to 1.5 microM. In addition to the effect on agonist-induced vasorelaxation, the Ca (2+) ionophore A23187-induced vasorelaxation was also inhibited or reversed by SA. However, SA had no effect on sodium nitroprusside-induced (guanylate cyclase activator) vasorelaxation. These data suggested that SA and its derivatives might be acting as inhibitors of nitric oxide synthesis in endothelium. At a concentration greater than 10 microM, SA induced contraction of intact but not denuded aorta which could be inhibited by prior treatment with indomethacin, a cyclooxygenase inhibitor.
CONCLUSIONS:
In summary, the results from this study showed that SA and its derivatives inhibited agonist-induced relaxation at lower concentrations and induced vasocontraction at higher concentrations. All the effects seen with SA were endothelium-dependent, however, through different mechanisms. |
|
| In vivo: |
| Biochem Pharmacol. 2012 Aug 15;84(4):507-12. | | β,β-Dimethylacrylshikonin exerts antitumor activity via Notch-1 signaling pathway in vitro and in vivo.[Pubmed: 22634048] | Dimethylacrylshikonin (DA) is a major component of Radix Lithospermum erythrorhizon and has various biological activities.We have investigated the inhibitory effect of Dimethylacrylshikonin on the growth of hepatocellular carcinoma in vitro and in vivo. Notch signaling plays a critical role in maintaining the balance between cell proliferation, differentiation and apoptosis. Hence, perturbed Notch signaling may contribute to tumorigenesis.
METHODS AND RESULTS:
In the present study, we evaluated whether Dimethylacrylshikonin could be an effective inhibitor on cell growth in human gastric cancer cell line, and also the molecular mechanisms. Using multiple cellular and molecular approaches such as MTT assay, colony formation assay, DAPI staining, flow cytometry, real-time PCR and Western blot analysis, we found that Dimethylacrylshikonin inhibited cell growth in a dose- and time-dependent manner. Biochemical analysis revealed the involvement of cell cycle regulated proteins in DA-mediated of G₀-G₁ arrest of SGC-7901 cells. Furthermore, Dimethylacrylshikonin treatment led to reduced Notch-1 activation, expression of Jagged-1 and its downstream target Hes-1 in vitro and in vivo.
CONCLUSIONS:
Our data demonstrated that Dimethylacrylshikonin is a potent inhibitor of progression of gastric cancer cells, which could be due to attenuation of Notch-1. We also suggest that Dimethylacrylshikonin could be further developed as a potential therapeutic agent for the treatment of gastric cancer. | | Int J Mol Med . 2015 Sep;36(3):685-97. | | Arnebin-1 promotes angiogenesis by inducing eNOS, VEGF and HIF-1α expression through the PI3K-dependent pathway[Pubmed: 26202335] | | Abstract
Arnebin-1, a naphthoquinone derivative, plays a crucial role in the wound healing properties of Zicao (a traditional wound healing herbal medicine). It has been noted that Arnebin-1, in conjunction with vascular endothelial growth factor (VEGF), exerts a synergistic pro-angiogenic effect on human umbilical vein endothelial cells (HUVECs) and accelerates the healing process of diabetic wounds. However, the mechanisms responsible for the pro-angiogenic effect of arnebin‑1 on HUVECs and its healing effect on diabetic wounds have not yet been fully elucidated. In this study, in an aim to elucidate these mechanisms of action of arnebin‑1, we investigated the effects of arnebin‑1 on the VEGF receptor 2 (VEGFR2) and the phosphoinositide 3-kinase (PI3K)‑dependent signaling pathways in HUVECs treated with VEGF by western blot analysis. The pro‑angiogenic effects of arnebin‑1 on HUVECs, including its effects on proliferation and migration, were evaluated by MTT assay, Transwell assay and tube formation assay in vitro. The expression levels of hypoxia-inducible factor (HIF)‑1α, endothelial nitric oxide synthase (eNOS) and VEGF were determined by western blot analysis in the HUVECs and wound tissues obtained from non‑diabetic and diabetic rats. CD31 expression in the rat wounds was evaluated by immunofluorescence staining. We found that the activation of the VEGFR2 signaling pathway induced by VEGF was enhanced by arnebin‑1. Arnebin‑1 promoted endothelial cell proliferation, migration and tube formation through the PI3K‑dependent pathway. Moreover, Arnebin‑1 significantly increased the eNOS, VEGF and HIF‑1α expression levels in the HUVECs and accelerated the healing of diabetic wounds through the PI3K‑dependent signaling pathway. CD31 expression was markedly enhanced in the wounds of diabetic rats treated with arnebin‑1 compared to the wounds of untreated diabetic rats. Therefore, the findings of the present study indicate that arnebin-1 promotes the wound healing process in diabetic rats by eliciting a pro-angiogenic response. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)