| In vitro: |
| J Nat Prod. 2016 Nov 23;79(11):2814-23. | | Phenanthrenes from Juncus inflexus with Antimicrobial Activity against Methicillin-Resistant Staphylococcus aureus.[Pubmed: 27808510 ] | The present study has focused on an investigation of the antibacterial effects of Juncus inflexus and the isolation and identification of its active compounds.
METHODS AND RESULTS:
Eleven phenanthrenes were isolated from a methanolic extract of the roots. Four compounds (jinflexins A-D, 1-4) are new natural products, while seven phenanthrenes [juncuenins A (5), B (6), and D (8), Juncusol (7), dehydrojuncuenins A (9) and B (11), and dehydroJuncusol (10)] were isolated for the first time from the plant. Jinflexin D (4) is a dimer with an unprecedented heptacyclic ring system. The absolute configurations of the new compounds were determined by TDDFT-ECD calculations, and their enantiomeric purity was checked by chiral HPLC analysis. Extracts of different polarity (n-hexane, dichloromethane, and ethyl acetate) were evaluated for their antimicrobial effects against methicillin-resistant Staphylococcus aureus, extended-spectrum β-lactamase (ESBL)-producing Citrobacter freundii, Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, multiresistant Acinetobacter baumannii, and Pseudomonas aeruginosa.
CONCLUSIONS:
The MIC values of the isolated compounds were determined by a microdilution method. Jinflexin B (2), Juncusol (7), juncuenin D (8), and dehydrojuncuenin B (11) showed significant activity (MIC value range 12.5-100 μg/mL) against MRSA strains. | | J Nat Med. 2015 Jul;69(3):421-6. | | Chemical constituents isolated from Juncus effusus induce cytotoxicity in HT22 cells.[Pubmed: 25794817 ] |
METHODS AND RESULTS:
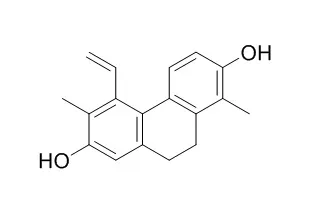
Effususol A (1), a new 9,10-dihydrophenanthrene, has been isolated from the medullae of Juncus effusus along with ten known compounds, effusol (2), dehydroeffusol (3), Juncusol (4), dehydroJuncusol (5), juncuenin B (6), dehydrojuncuenin B (7), juncuenin D (8), luteolin (9), luteolin 5-methyl ether (10), and 4-hydroxy-2,3-dimethyl-2-nonen-4-olide (11).
CONCLUSIONS:
The structure of 1 was elucidated on the basis of spectroscopic data. 2, 4, 6, 7, and 8 have induced caspase-3-mediated cytotoxicity in HT22 cells. |
|
| In vivo: |
| Nat Prod Res. 2012;26(13):1234-9. | | Phenanthrenes from Juncus effusus with anxiolytic and sedative activities.[Pubmed: 22017742 ] | Eight phenanthrenes, 7-carboxy-2-hydroxy-1-methyl-5-vinyl-phenanthrene (1); 2,7-dihydroxy-1-methyl-5-aldehyde-9,10-dihydrophenanthrene (2); dehydroeffusol (3); dehydroJuncusol (4); 7-carboxy-2-hydroxy-1-methyl-5-vinyl-9,10-dihydrophenanthrene (5); 8-carboxy-2-hydroxy-1-methyl-5-vinyl-9,10-dihydrophenanthrene (6); effusol (7) and Juncusol (8), were isolated from the aerial part of Juncus effusus.
METHODS AND RESULTS:
Compounds 1 and 2 were identified as new constituents. Compounds 7 and 8 showed anxiolytic and sedative activities. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)