| Animal Research: |
| Biol Pharm Bull. 2014;37(5):858-64. | | Anti-allodynic and neuroprotective effects of koumine, a Benth alkaloid, in a rat model of diabetic neuropathy.[Pubmed: 24790009 ] | Diabetic neuropathy is characterized by progressive degeneration of nerve fibers associated with diabetes mellitus. Antidepressants and anticonvulsants are the mainstay of pharmacological treatment, but are often limited in effectiveness against the core clinical feature of pain. In the current study, we examined the potential effects of Koumine, a Gelsemium elegans Benth alkaloid, using a rat model of diabetic neuropathy.
METHODS AND RESULTS:
Rats were administered intraperitoneally a single dose of streptozocin (60 mg/kg) to induce type 1 diabetes. Koumine was given at a dose range of 0.056-7 mg/kg subcutaneously for one week starting 3 weeks after streptozocin adminstration. Behavioral responses to mechanical stimuli were evaluated every day after streptozocin injection. At 4 weeks after streptozocin injection, sensory nerve conduction velocity (SNCV) and morphological alternation of sciatic nerves were assessed by electron microscopy. Diabetic rats developed mechanical hyperalgesia within 3 weeks after streptozocin injection and exhibited reduced SNCV and impaired myelin/axonal structure. Koumine treatment of diabetic rats decreased neuropathic pain behavior as early as after the first administration. At a dose of 7 mg/kg, Koumine was more effective than gabapentin (100 mg/kg), and decreased mechanical sensitivity threshold to a level comparable to healthy control.
CONCLUSIONS:
Repeated treatment of Koumine significantly reduced the damage to axon and myelin sheath of the sciatic nerve and increased SNCV, without affecting body weight and blood glucose. These findings encourage the use of Koumine in the treatment of diabetic neuropathy. |
|
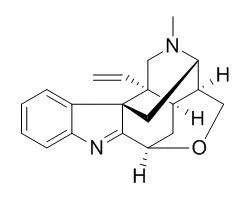
| Structure Identification: |
| J Asian Nat Prod Res. 2013;15(1):46-52. | | Four new koumine metabolites in rat liver microsomes.[Pubmed: 23323600] |
METHODS AND RESULTS:
Four new metabolites M-1 [1,2,18,19-tetradehydro-4-demethyl-3,17-epoxy-7,20(2H,19H)-cyclovobasan], M-2 [1,2,4,21,18,19-hexadehydro-4-demethyl-3,17-epoxy-7,20(2H,19H)-cyclovobasan], M-3 [1,2,18,19-tetradehydro-4-demethyl-4-formaldehyde-3,17-epoxy-7,20(2H,19H)-cyclovobasan], and M-4 [1,2,4,21,18,19-hexadehydro-4-demethyl-4-oxy-3,17-epoxy-7,20(2H,19H)-cyclovobasan] were isolated from the chloroform extract of Koumine incubated with phenobarbital-treated rat liver microsomes. The structures of M-1, M-2, M-3, and M-4 were elucidated by spectroscopic methods including ESI-TOF-MS, 1D, and 2D NMR experiments.
CONCLUSIONS:
The metabolic pathway of Koumine was proposed. The cytotoxic activities between Koumine and its metabolites were also compared in the A549 cell line. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)