| Kinase Assay: |
| Scientific Reports, 2016, 6:36543. | | Kukoamine A inhibits human glioblastoma cell growth and migration through apoptosis induction and epithelial-mesenchymal transition attenuation.[Reference: WebLink] | Cortex lycii radicis is the dried root bark of Lycium chinense, a traditional Chinese herb used in multiple ailments. The crude extract of Cortex lycii radicis has growth inhibition effect on GBM cells. Kukoamine A (KuA) is a spermine alkaloid derived from it. KuA possesses antioxidant, anti-inflammatory activities, but its anticancer activity is unknown.
METHODS AND RESULTS:
In this study, the growth and migration inhibition effect of KuA on human GBM cells and the possible mechanism of its activity were investigated. After KuA treatment, proliferation and colony formation of GBM cells were decreased significantly; apoptotic cells were increased; the cell cycle was arrested G0/G1 phase; the migration and invasion were decreased, the growth of tumors initiated from GBM cells was inhibited significantly; the expressions of 5-Lipoxygenase (5-LOX) were decreased, apoptotic proteins, Bax and caspase-3 were increased, and antiapoptotic protein Bcl-2 was decreased significantly; The expressions of CCAAT/enhancer binding protein β (C/EBPβ), N-cadherin, vimentin, twist and snail+slug were decreased significantly, while the expression of E-cadherin was increased significantly in KuA treated GBM cells and tumor tissues.
CONCLUSIONS:
KuA inhibited human glioblastoma cell growth and migration in vitro and in vivo through apoptosis induction and epithelial-mesenchymal transition attenuation by downregulating expressions of 5-LOX and C/EBPβ. |
|
| Animal Research: |
| Neurotoxicity Research, 2016, 31(2):259. | | Kukoamine A Prevents Radiation-Induced Neuroinflammation and Preserves Hippocampal Neurogenesis in Rats by Inhibiting Activation of NF-κB and AP-1.[Reference: WebLink] | Impaired hippocampal neurogenesis and neuroinflammation are involved in the pathogenesis of radiation-induced brain injury. Kukoamine A (KuA) was demonstrated to have neuroprotective effects through inhibiting oxidative stress and apoptosis after whole-brain irradiation (WBI) in rats. The aim of this study was to investigate whether administration of KuA would prevent radiation-induced neuroinflammation and the detrimental effect on hippocampal neurogenesis.
METHODS AND RESULTS:
For this study, male Wistar rats received either sham irradiation or WBI (30 Gy single dose of X-rays) followed by the immediate injection of either KuA or vehicle intravenously. The dose of KuA was 5, 10, and 20 mg/kg, respectively. The levels of pro-inflammatory cytokines were assayed by ELISA kits. The newborn neurons were detected by 5-bromo-2-deoxyuridine (BrdU)/neuronal nuclei (NeuN) double immunofluorescence. Microglial activation was measured by Iba-1 immunofluorescence. The expression of Cox-2 and the activation of nuclear factor κB (NF-κB), activating protein 1(AP-1), and PPARδ were evaluated by western blot. WBI led to a significant increase in the level of TNF-α, IL-1β, and Cox-2, and it was alleviated by KuA administration. KuA attenuated microglial activation in rat hippocampus after WBI. Neurogenesis impairment induced by WBI was ameliorated by KuA. Additionally, KuA alleviated the increased translocation of NF-κB p65 subunit and phosphorylation of c-jun induced by WBI and elevated the expression of PPARδ.
CONCLUSIONS:
These data indicate that KuA could ameliorate the neuroinflammatory response and protect neurogenesis after WBI, partially through regulating the activation of NF-κB, AP-1, and PPARδ. | | Biomedicine & Pharmacotherapy, 2017, 89:536-543. | | Kukoamine A attenuates insulin resistance and fatty liver through downregulation of Srebp-1c.[Reference: WebLink] | Nonalcoholic fatty liver disease (NAFLD) refers to a pathological condition of hepatic steatosis. Insulin resistance is believed to be the key mechanism mediating initial accumulation of fat in the liver, resulting in hepatic steatosis. Kukoamine A (KuA), a spermine alkaloid, is a major bioactive component extracted from the root barks of Lycium chinense (L. chinense) Miller.
METHODS AND RESULTS:
In the current study, we aimed to explore the possible effect of KuA on insulin resistance and fatty liver. We showed that KuA significantly inhibited the increase of fasting blood glucose level and insulin level, and the glucose levels in response to glucose and insulin load in HFD-fed mice, which was in a dose-dependent manner. KuA dose-dependently decreased the histological injury of liver, levels of hepatic triglyceride (TG), and serum AST and ALT activities in HFD-fed mice. The increase of serum levels of TNFɑ, IL-1β, IL-6 and C reactive protein in HFD-fed mice was inhibited by KuA. HFD feeding-induced increase of hepatic expression of Srebp-1c and its target genes, including fatty acid synthase (FAS) and acetyl CoA carboxylase 1 (ACC1), was significantly inhibited by KuA. Moreover, upregulation of Srebp-1c notably inhibited KuA-induced improvement of insulin-stimulated glucose uptake, decrease of lipid accumulation and H2O2 level in palmitic acid-treated AML-12 cells.
CONCLUSIONS:
In conclusion, we reported that KuA inhibited Srebp-1c and downstream genes expression and resulted in inhibition of lipid accumulation, inflammation, insulin resistance and oxidative stress.
Overall, our results provide a better understanding of the pharmacological activities of KuA against insulin resistance and hepatic steatosis. |
|


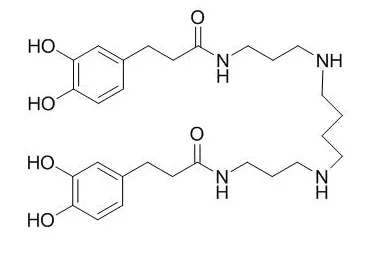



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)